Get the free qualified investigator undertaking form
Show details
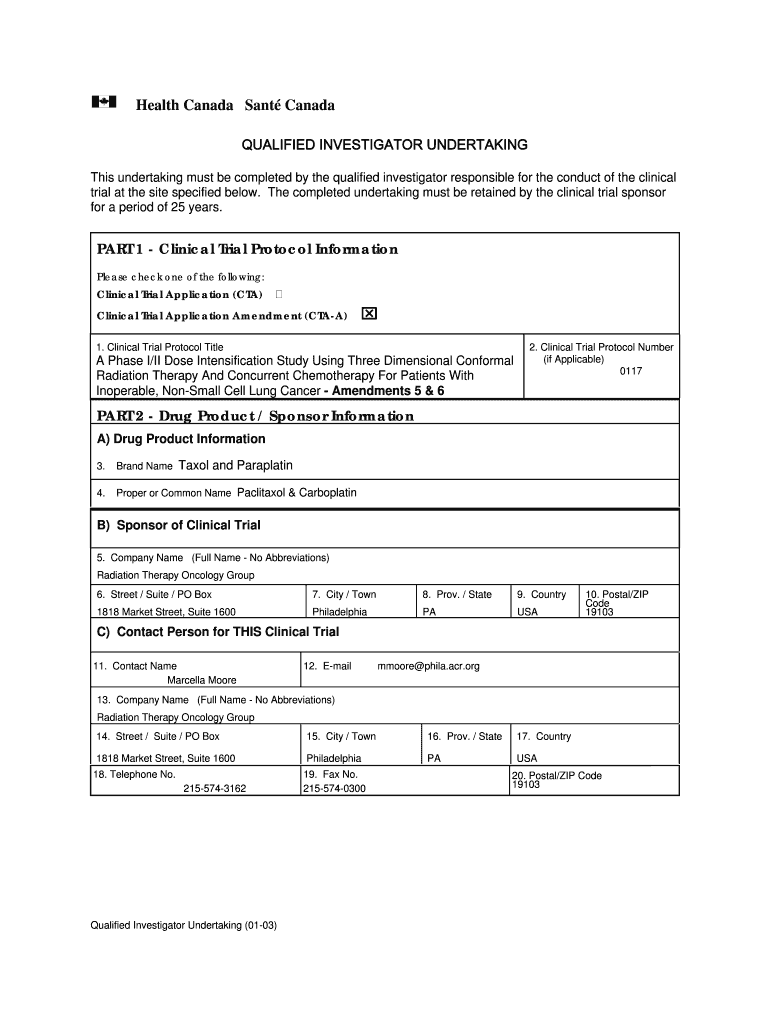
Health Canada Sent Canada QUALIFIED INVESTIGATOR UNDERTAKING This undertaking must be completed by the qualified investigator responsible for the conduct of the clinical trial at the site specified
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign the qualified investigator undertaking form is a document with regulatory standards and protocols during the study msockid 22b6b242be4869a72a62a600bf7368f6

Edit your investigator undertaking form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your qualified investigator undertaking form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing qiu form online
To use our professional PDF editor, follow these steps:
1
Create an account. Begin by choosing Start Free Trial and, if you are a new user, establish a profile.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit ches00900e form. Rearrange and rotate pages, insert new and alter existing texts, add new objects, and take advantage of other helpful tools. Click Done to apply changes and return to your Dashboard. Go to the Documents tab to access merging, splitting, locking, or unlocking functions.
4
Save your file. Select it from your list of records. Then, move your cursor to the right toolbar and choose one of the exporting options. You can save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud, among other things.
pdfFiller makes dealing with documents a breeze. Create an account to find out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out qualified investigator undertaking form

How to fill out qualified investigator undertaking form:
01
Begin by reading the instructions provided with the form carefully.
02
Fill in your personal information accurately and completely, including your name, contact details, and any relevant identification numbers.
03
Provide details of your qualifications and experience as an investigator, including any certifications or licenses you hold.
04
Indicate your understanding of the ethical and legal responsibilities associated with being a qualified investigator.
05
If applicable, include any references or endorsements from previous clients or employers.
06
Sign and date the form to confirm your commitment to upholding the standards and responsibilities of a qualified investigator.
Who needs qualified investigator undertaking form:
01
Individuals who wish to work as professional investigators and want to demonstrate their qualifications and commitment to ethical practices.
02
Companies or organizations that require proof of an investigator's expertise and adherence to professional standards.
03
Clients who want assurance that the investigator they hire has the necessary skills and knowledge to carry out the required tasks.
Fill
form
: Try Risk Free






People Also Ask about
Does Canada use a 1572?
FDA Form 1572 for Canadian Sites It states that the clinical investigator will: Conduct the study ing to the protocol. Personally conduct/supervise the study. Ensure proper consent and IRB review.
What is a Qiu form?
An undertaking must be completed by the qualified investigator responsible for the conduct of the clinical trial at the site specified below. The completed undertaking must be retained by the clinical trial sponsor for a period of 25 years.
What is the purpose of the Form 1572?
The Statement of Investigator, Form FDA 1572 (1572), is an agreement signed by the Investigator to provide certain information to the sponsor and assure that he/she will comply with FDA regulations related to the conduct of a clinical investigation of an investigational drug or biologic.
What is the undertaking form 1572?
The Statement of Investigator, Form FDA 1572 is an agreement (one‐sided contract) signed by a clinical trial investigator to provide certain information to the clinical trial sponsor, and to assure the sponsor that he/she will comply with US FDA regulations related to the conduct of a clinical investigation of an
What is an FDA Form 1572 purpose and use?
The investigator's signature on this form constitutes the investigator's affirmation that he or she is qualified to conduct the clinical investigation and constitutes the investigator's written commitment to abide by FDA regulations in the conduct of the clinical investigations.
Who can be listed as a qualified investigator?
05.001 qualified investigator means the person responsible to the sponsor for the conduct of the clinical trial at a clinical trial site, who is entitled to provide health care under the laws of the province where that clinical trial site is located, and who is(a) in the case of a clinical trial respecting a drug to be
What is a qualified undertaking form?
What is a qualified investigator undertaking form? An undertaking must be completed by the qualified investigator responsible for the conduct of the clinical trial at the site specified below. The completed undertaking must be retained by the clinical trial sponsor for a period of 25 years.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I send qualified investigator undertaking form for eSignature?
When you're ready to share your qualified investigator undertaking form, you can swiftly email it to others and receive the eSigned document back. You may send your PDF through email, fax, text message, or USPS mail, or you can notarize it online. All of this may be done without ever leaving your account.
How do I execute qualified investigator undertaking form online?
pdfFiller makes it easy to finish and sign qualified investigator undertaking form online. It lets you make changes to original PDF content, highlight, black out, erase, and write text anywhere on a page, legally eSign your form, and more, all from one place. Create a free account and use the web to keep track of professional documents.
How do I fill out the qualified investigator undertaking form form on my smartphone?
You can quickly make and fill out legal forms with the help of the pdfFiller app on your phone. Complete and sign qualified investigator undertaking form and other documents on your mobile device using the application. If you want to learn more about how the PDF editor works, go to pdfFiller.com.
What is qualified investigator undertaking form?
The qualified investigator undertaking form is a document used in clinical research that outlines the responsibilities and commitments of a qualified investigator in ensuring compliance with regulatory standards and protocols during the study.
Who is required to file qualified investigator undertaking form?
Individuals acting as qualified investigators for clinical trials or studies are required to file the qualified investigator undertaking form to confirm their qualifications and commitment to adhere to study protocols.
How to fill out qualified investigator undertaking form?
To fill out the qualified investigator undertaking form, the investigator should provide their personal details, confirm their qualifications, outline their experience related to the study, and sign to indicate their commitment to follow regulatory requirements and protocol.
What is the purpose of qualified investigator undertaking form?
The purpose of the qualified investigator undertaking form is to ensure that qualified investigators acknowledge their responsibilities, comply with ethical standards, and commit to conducting clinical trials with integrity and in accordance with regulatory requirements.
What information must be reported on qualified investigator undertaking form?
The information that must be reported on the qualified investigator undertaking form typically includes the investigator's name and contact information, qualifications, relevant experience, the nature of the study, and a signed declaration of compliance with applicable regulations.
Fill out your qualified investigator undertaking form online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Qualified Investigator Undertaking Form is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















