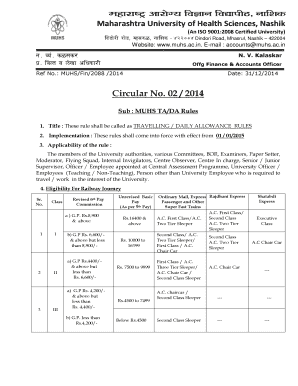
Get the free FDAaudit.doc - uab
Show details
Project dominant din Fondue Social European print Program Operational Sectorial Dezvoltarea Resurselor Humane 20072013. Invested n amend ! FONDUE SOCIAL EUROPEAN Program Operational Sectorial Dezvoltarea
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign fdaauditdoc - uab

Edit your fdaauditdoc - uab form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your fdaauditdoc - uab form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing fdaauditdoc - uab online
To use our professional PDF editor, follow these steps:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Upload a file. Select Add New on your Dashboard and upload a file from your device or import it from the cloud, online, or internal mail. Then click Edit.
3
Edit fdaauditdoc - uab. Add and replace text, insert new objects, rearrange pages, add watermarks and page numbers, and more. Click Done when you are finished editing and go to the Documents tab to merge, split, lock or unlock the file.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
With pdfFiller, it's always easy to work with documents. Try it out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out fdaauditdoc - uab

How to fill out fdaauditdoc?
01
Start by gathering all necessary information and documents related to the FDA audit. This may include previous audit reports, compliance records, and other relevant documentation.
02
Review the fdaauditdoc form carefully to understand the required information and sections to be filled out. Familiarize yourself with the format and any specific instructions provided.
03
Begin filling out the form by entering the necessary details in the designated fields. This may include your organization's name, address, contact information, and any unique identifiers or registration numbers.
04
Provide a brief overview or summary of your organization's activities and operations relevant to the FDA audit. This helps provide context and background information for the auditors.
05
Pay close attention to the questions or prompts within the fdaauditdoc form. Answer each question accurately and honestly, providing as much detail as possible. If a question is not applicable, indicate so clearly or provide a brief explanation.
06
Include any supporting documentation or evidence as required by the form. This may include records of quality control processes, production procedures, or any other relevant documentation that showcases compliance with FDA regulations.
07
Double-check all the information you have entered to ensure accuracy and completeness. Review for any spelling or typographical errors that could potentially affect the clarity or interpretation of your responses.
08
If necessary, seek clarification or guidance from a compliance officer or regulatory expert within your organization to ensure compliance with FDA regulations and accuracy of the fdaauditdoc.
Who needs fdaauditdoc?
01
Organizations or businesses involved in the production, manufacturing, or distribution of products regulated by the FDA may need to fill out the fdaauditdoc. This can include pharmaceutical companies, medical device manufacturers, food and beverage processors, and cosmetic producers.
02
Compliance officers or regulatory affairs professionals within these organizations are typically responsible for completing and submitting the fdaauditdoc. They ensure that all relevant information and documentation are accurately compiled and provided to the FDA during audits.
03
Organizations seeking FDA approval or compliance certification for new products or facilities may also need to complete the fdaauditdoc as part of the regulatory approval process. This helps the FDA assess the organization's adherence to regulatory guidelines and determine its eligibility for compliance certification.
It is important to refer to the specific guidelines and requirements outlined by the FDA or relevant regulatory authorities to determine if the fdaauditdoc is needed in a particular situation.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I execute fdaauditdoc - uab online?
pdfFiller has made it simple to fill out and eSign fdaauditdoc - uab. The application has capabilities that allow you to modify and rearrange PDF content, add fillable fields, and eSign the document. Begin a free trial to discover all of the features of pdfFiller, the best document editing solution.
How do I edit fdaauditdoc - uab on an Android device?
With the pdfFiller Android app, you can edit, sign, and share fdaauditdoc - uab on your mobile device from any place. All you need is an internet connection to do this. Keep your documents in order from anywhere with the help of the app!
How do I complete fdaauditdoc - uab on an Android device?
On an Android device, use the pdfFiller mobile app to finish your fdaauditdoc - uab. The program allows you to execute all necessary document management operations, such as adding, editing, and removing text, signing, annotating, and more. You only need a smartphone and an internet connection.
What is fdaauditdoc?
fdaauditdoc is a document that contains information related to audits conducted by the Food and Drug Administration (FDA).
Who is required to file fdaauditdoc?
All manufacturers, distributors, and importers of FDA-regulated products are required to file fdaauditdoc.
How to fill out fdaauditdoc?
fdaauditdoc can be filled out electronically through the FDA's online portal or by submitting a physical copy to the FDA.
What is the purpose of fdaauditdoc?
The purpose of fdaauditdoc is to ensure transparency and compliance with FDA regulations regarding audits of regulated products.
What information must be reported on fdaauditdoc?
Information such as the date of the audit, the name of the auditor, the findings of the audit, and any corrective actions taken must be reported on fdaauditdoc.
Fill out your fdaauditdoc - uab online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Fdaauditdoc - Uab is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.