Get the free Clinical Trials and Volunteer Studies at Fred Hutchinson ...
Show details
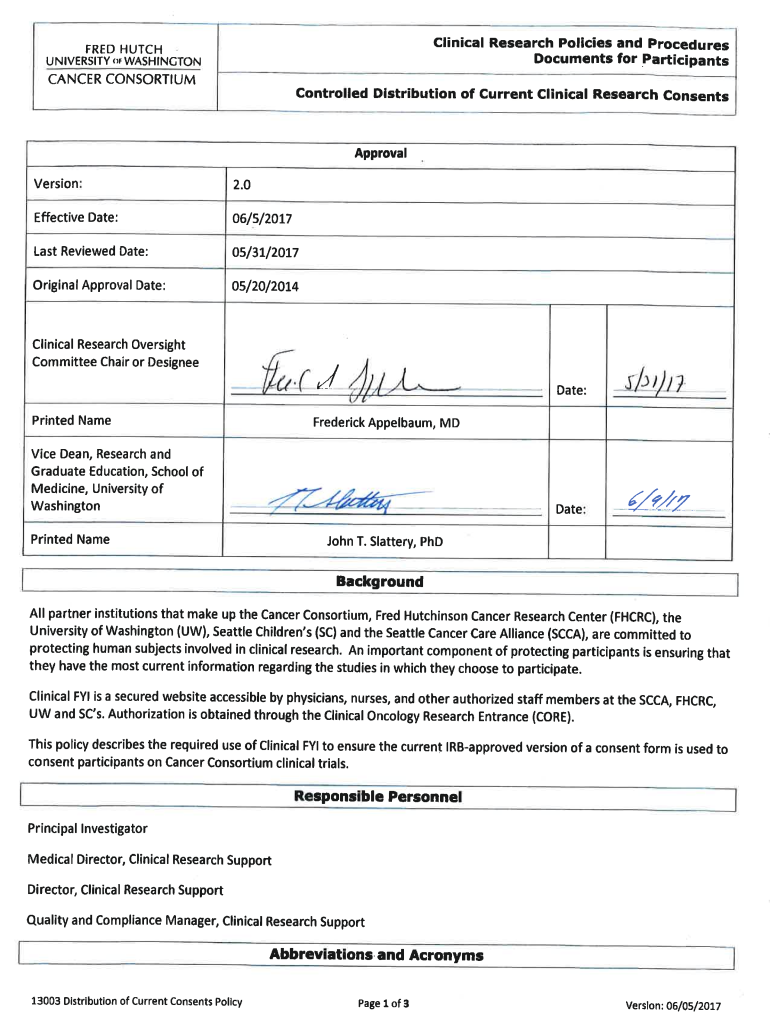
Clinical Research Policies and Procedures Documents for Participants Controlled Distribution of Current Clinical Research ConsentsCORE: Clinical Oncology Research Entrance CRS: Clinical Research Support
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign clinical trials and volunteer

Edit your clinical trials and volunteer form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your clinical trials and volunteer form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit clinical trials and volunteer online
Use the instructions below to start using our professional PDF editor:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Upload a document. Select Add New on your Dashboard and transfer a file into the system in one of the following ways: by uploading it from your device or importing from the cloud, web, or internal mail. Then, click Start editing.
3
Edit clinical trials and volunteer. Text may be added and replaced, new objects can be included, pages can be rearranged, watermarks and page numbers can be added, and so on. When you're done editing, click Done and then go to the Documents tab to combine, divide, lock, or unlock the file.
4
Save your file. Select it from your records list. Then, click the right toolbar and select one of the various exporting options: save in numerous formats, download as PDF, email, or cloud.
Dealing with documents is always simple with pdfFiller. Try it right now
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out clinical trials and volunteer

How to fill out clinical trials and volunteer
01
Research and gather information on clinical trials and volunteer opportunities at reputable organizations or institutions.
02
Determine your eligibility by reviewing the specific requirements and criteria set by the clinical trial or volunteer program.
03
Consult with a healthcare professional or your primary care physician to assess if participating in a clinical trial is suitable for your medical condition or if volunteering aligns with your interests and availability.
04
Fill out the application forms provided by the organization running the clinical trial or volunteer program.
05
Provide accurate and detailed personal information, medical history, and any relevant documentation as requested.
06
Attend any required interviews, orientations, or training sessions as part of the application process.
07
Follow all instructions and guidelines provided throughout the clinical trial or volunteer assignment.
08
Keep track of any scheduled appointments, medication or treatment schedules, and follow-up procedures as required.
09
Maintain open communication with the organization or institution coordinating the clinical trial or volunteer program, reporting any concerns or changes in your health or availability.
10
Upon completion of the clinical trial or volunteer assignment, provide feedback to the organization or institution to support future improvements and research advancements.
Who needs clinical trials and volunteer?
01
Individuals with certain medical conditions, diseases, or disabilities may need clinical trials to access potential treatments or therapies that are not yet widely available or approved.
02
Researchers and scientists conducting clinical trials need volunteers to participate in order to gather essential data and evaluate the safety and effectiveness of new medications, procedures, or interventions.
03
Individuals interested in contributing to the advancement of medical research and development may volunteer to support various aspects of healthcare studies, such as data collection, administrative tasks, patient support, or raising awareness.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I edit clinical trials and volunteer in Chrome?
Add pdfFiller Google Chrome Extension to your web browser to start editing clinical trials and volunteer and other documents directly from a Google search page. The service allows you to make changes in your documents when viewing them in Chrome. Create fillable documents and edit existing PDFs from any internet-connected device with pdfFiller.
Can I create an electronic signature for the clinical trials and volunteer in Chrome?
Yes. You can use pdfFiller to sign documents and use all of the features of the PDF editor in one place if you add this solution to Chrome. In order to use the extension, you can draw or write an electronic signature. You can also upload a picture of your handwritten signature. There is no need to worry about how long it takes to sign your clinical trials and volunteer.
How do I fill out clinical trials and volunteer using my mobile device?
Use the pdfFiller mobile app to fill out and sign clinical trials and volunteer on your phone or tablet. Visit our website to learn more about our mobile apps, how they work, and how to get started.
What is clinical trials and volunteer?
Clinical trials are research studies that test new drugs, treatments, or medical devices in people to evaluate their effectiveness and safety. Volunteers are individuals who choose to participate in these trials.
Who is required to file clinical trials and volunteer?
Researchers, study sponsors, or organizations conducting clinical trials are required to file information about the trials and report on the volunteers involved.
How to fill out clinical trials and volunteer?
Filling out clinical trials and volunteer information typically involves submitting a protocol outlining the trial's objectives, designs, and methodologies through a designated registry or regulatory agency, as well as providing informed consent documents for volunteers.
What is the purpose of clinical trials and volunteer?
The purpose of clinical trials is to evaluate the safety and effectiveness of new treatments or interventions, while volunteers help facilitate this research by providing data on how these treatments perform in a human population.
What information must be reported on clinical trials and volunteer?
Information that must be reported includes the trial's objectives, eligibility criteria for volunteers, study procedures, potential risks and benefits, and the informed consent process.
Fill out your clinical trials and volunteer online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Clinical Trials And Volunteer is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.


















