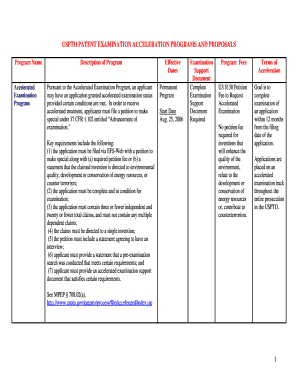
Get the free What FDA Expects in your Submissions: Biologics & Drugs
Show details
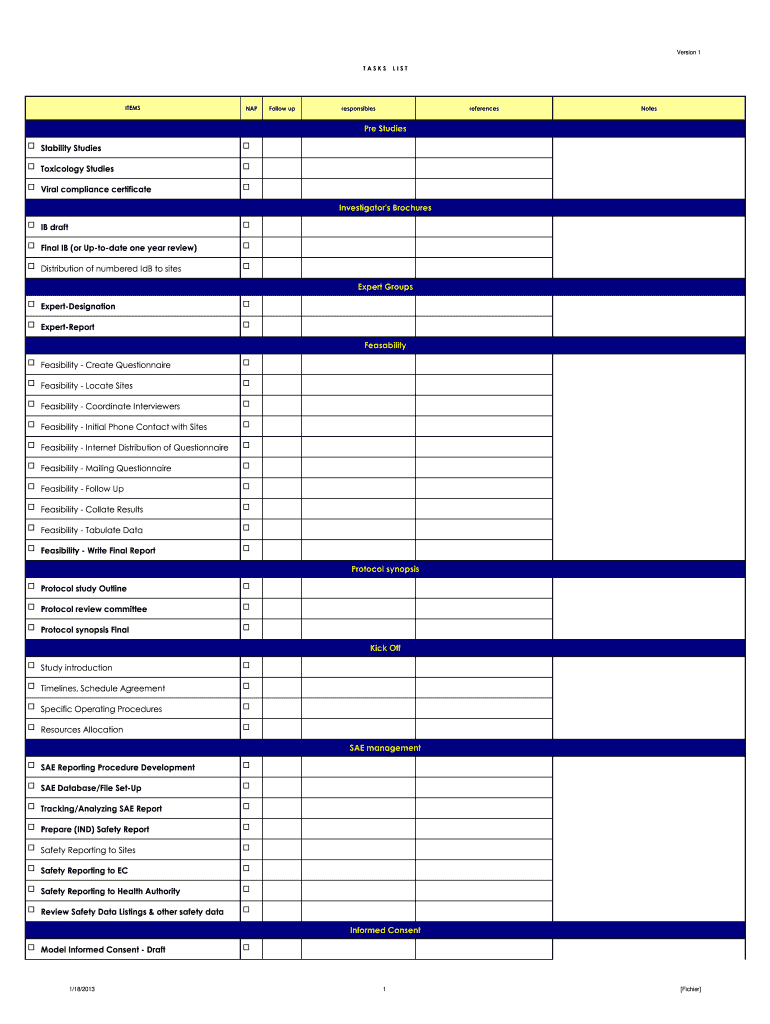
Version 1 TASKSITEMSNAPFollow upLISTresponsiblesreferencesNotesPre Studies Stability Studies Toxicology Studies Viral compliance certificate Investigator's Brochures IB draft Final IB (or Update one
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign what fda expects in

Edit your what fda expects in form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your what fda expects in form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit what fda expects in online
Follow the steps below to benefit from a competent PDF editor:
1
Log in. Click Start Free Trial and create a profile if necessary.
2
Upload a file. Select Add New on your Dashboard and upload a file from your device or import it from the cloud, online, or internal mail. Then click Edit.
3
Edit what fda expects in. Rearrange and rotate pages, add new and changed texts, add new objects, and use other useful tools. When you're done, click Done. You can use the Documents tab to merge, split, lock, or unlock your files.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
pdfFiller makes working with documents easier than you could ever imagine. Create an account to find out for yourself how it works!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out what fda expects in

How to fill out what fda expects in
01
To fill out what FDA expects, follow these steps:
02
Familiarize yourself with FDA regulations and guidelines for the specific product or industry you are working in. These can be found on the FDA website.
03
Understand the specific requirements and expectations outlined by the FDA for your product. Determine what information needs to be included in the submission.
04
Prepare all necessary documentation and data relevant to your product. This may include safety data, manufacturing processes, labeling information, and any clinical or preclinical trial results.
05
Organize and format the information according to FDA guidelines. Make sure to include all required sections and data fields.
06
Fill out the appropriate FDA forms or online submission portals. Pay careful attention to accuracy and completeness of the information provided.
07
Review and double-check all information before submission. Ensure that all requirements have been met and that the submission is error-free.
08
Submit the completed documentation to the FDA. Follow their specified submission process, which may include electronic submission, physical mail, or a combination of both.
09
Keep a record of the submission for your records. This can be helpful for future reference and in case of any questions or follow-up from the FDA.
10
Monitor the status of your submission. Depending on the FDA's processing times, you may receive feedback or requests for additional information. Respond promptly and fully to any FDA inquiries.
11
Stay updated on any changes or updates to FDA regulations and guidelines that may impact your product's compliance. Maintain compliance with ongoing FDA requirements.
Who needs what fda expects in?
01
Anyone who is involved in the manufacturing, distribution, or importation of products regulated by the FDA needs to understand and fulfill what FDA expects.
02
This includes:
03
- Pharmaceutical companies
04
- Medical device manufacturers
05
- Biotechnology firms
06
- Food and dietary supplement manufacturers
07
- Cosmetic companies
08
- Tobacco product manufacturers
09
Compliance with FDA expectations is crucial for ensuring product safety, quality, and legality. Failure to meet FDA requirements can result in regulatory action, including product recalls, fines, or legal repercussions.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I edit what fda expects in from Google Drive?
Simplify your document workflows and create fillable forms right in Google Drive by integrating pdfFiller with Google Docs. The integration will allow you to create, modify, and eSign documents, including what fda expects in, without leaving Google Drive. Add pdfFiller’s functionalities to Google Drive and manage your paperwork more efficiently on any internet-connected device.
How can I send what fda expects in for eSignature?
Once you are ready to share your what fda expects in, you can easily send it to others and get the eSigned document back just as quickly. Share your PDF by email, fax, text message, or USPS mail, or notarize it online. You can do all of this without ever leaving your account.
How do I edit what fda expects in straight from my smartphone?
The best way to make changes to documents on a mobile device is to use pdfFiller's apps for iOS and Android. You may get them from the Apple Store and Google Play. Learn more about the apps here. To start editing what fda expects in, you need to install and log in to the app.
What is what FDA expects in?
FDA expects a comprehensive submission of information that complies with regulatory standards for various products.
Who is required to file what FDA expects in?
Manufacturers, importers, and authorized representatives of regulated products are required to file what FDA expects in.
How to fill out what FDA expects in?
To fill out what FDA expects in, follow the specific guidelines provided by the FDA, including accurate completion of forms and submission of required documentation.
What is the purpose of what FDA expects in?
The purpose is to ensure the safety, efficacy, and quality of products regulated by the FDA through systematic reporting.
What information must be reported on what FDA expects in?
Information regarding product composition, manufacturing processes, labeling, and safety data must be reported.
Fill out your what fda expects in online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

What Fda Expects In is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.