Get the free Cleanroom, Microbiology and Sterility Assurance Practices
Show details
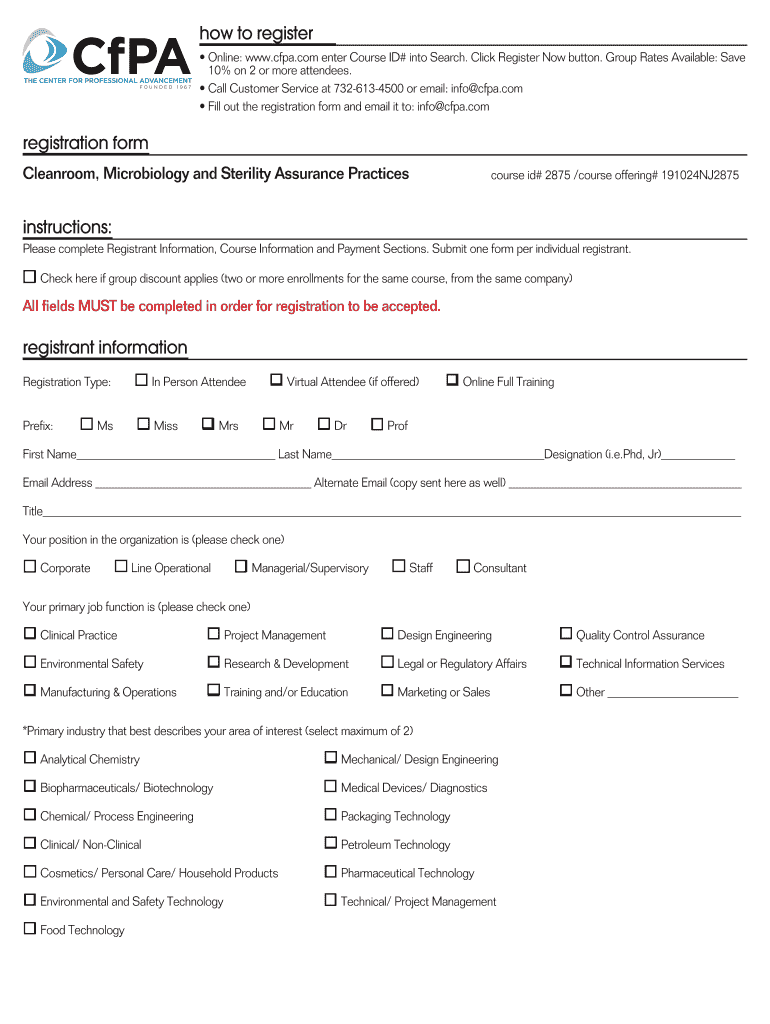
Completely Revised! Clean room, Microbiology and Sterility Assurance Practices Drug and Device Manufacturers October 2425, 2019 New Jersey Charity Ogunsanya, (Owner/CEO), Pharmabiodevice Consulting
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign cleanroom microbiology and sterility

Edit your cleanroom microbiology and sterility form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your cleanroom microbiology and sterility form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing cleanroom microbiology and sterility online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Log in to account. Click Start Free Trial and register a profile if you don't have one.
2
Upload a document. Select Add New on your Dashboard and transfer a file into the system in one of the following ways: by uploading it from your device or importing from the cloud, web, or internal mail. Then, click Start editing.
3
Edit cleanroom microbiology and sterility. Add and change text, add new objects, move pages, add watermarks and page numbers, and more. Then click Done when you're done editing and go to the Documents tab to merge or split the file. If you want to lock or unlock the file, click the lock or unlock button.
4
Save your file. Select it in the list of your records. Then, move the cursor to the right toolbar and choose one of the available exporting methods: save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud.
pdfFiller makes dealing with documents a breeze. Create an account to find out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out cleanroom microbiology and sterility

How to fill out cleanroom microbiology and sterility
01
Ensure that you are wearing the appropriate personal protective equipment (PPE), including a cleanroom suit, gloves, and a face mask.
02
Thoroughly clean and disinfect all surfaces in the cleanroom area using appropriate disinfectants.
03
Prepare the necessary materials and equipment for microbiology and sterility testing, including culture media, test samples, and sterilized instruments.
04
Follow aseptic techniques when handling the test samples and performing microbiology experiments.
05
Use sterile techniques and equipment to minimize the risk of contamination.
06
Perform appropriate microbiological tests, such as environmental monitoring, microbial identification, and microbial enumeration.
07
Follow standardized protocols and guidelines for sterility testing to ensure accurate and reliable results.
08
Maintain proper documentation and records of all procedures and results.
09
Regularly validate the cleanroom environment and equipment to ensure compliance with sterility standards.
10
Monitor and control the cleanroom environment, including temperature, humidity, and particulate levels, to prevent microbial growth and maintain sterility.
Who needs cleanroom microbiology and sterility?
01
Cleanroom microbiology and sterility are needed by various industries and sectors, including:
02
- Pharmaceutical companies to ensure the safety and effectiveness of their products
03
- Biotechnology companies to maintain the sterility of their laboratory environments
04
- Healthcare facilities to prevent healthcare-associated infections
05
- Food and beverage manufacturers to ensure product safety and quality
06
- Research laboratories to safeguard against contamination and maintain the integrity of their experiments
07
- Medical device manufacturers to ensure the sterility of their products
08
- Aerospace industry to maintain cleanliness in the production of sensitive components
09
- Electronic industry to prevent contamination of electronic devices.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
Can I sign the cleanroom microbiology and sterility electronically in Chrome?
As a PDF editor and form builder, pdfFiller has a lot of features. It also has a powerful e-signature tool that you can add to your Chrome browser. With our extension, you can type, draw, or take a picture of your signature with your webcam to make your legally-binding eSignature. Choose how you want to sign your cleanroom microbiology and sterility and you'll be done in minutes.
Can I create an eSignature for the cleanroom microbiology and sterility in Gmail?
You can easily create your eSignature with pdfFiller and then eSign your cleanroom microbiology and sterility directly from your inbox with the help of pdfFiller’s add-on for Gmail. Please note that you must register for an account in order to save your signatures and signed documents.
How can I fill out cleanroom microbiology and sterility on an iOS device?
In order to fill out documents on your iOS device, install the pdfFiller app. Create an account or log in to an existing one if you have a subscription to the service. Once the registration process is complete, upload your cleanroom microbiology and sterility. You now can take advantage of pdfFiller's advanced functionalities: adding fillable fields and eSigning documents, and accessing them from any device, wherever you are.
What is cleanroom microbiology and sterility?
Cleanroom microbiology is the study of microorganisms in controlled environments to ensure cleanliness and minimize contamination. Sterility refers to the state of being free from all living microorganisms, which is crucial in settings like pharmaceuticals and biotechnology.
Who is required to file cleanroom microbiology and sterility?
Organizations that operate cleanrooms, such as pharmaceutical companies, biotechnology firms, and medical device manufacturers, are required to file reports on cleanroom microbiology and sterility.
How to fill out cleanroom microbiology and sterility?
To fill out cleanroom microbiology and sterility reports, organizations must document relevant microbial testing results, environmental monitoring data, and control measures taken to maintain sterility.
What is the purpose of cleanroom microbiology and sterility?
The purpose of cleanroom microbiology and sterility is to ensure the safety and integrity of products being manufactured in sterile environments, preventing contamination that could compromise product quality.
What information must be reported on cleanroom microbiology and sterility?
Reports must include microbial counts, results of sterility tests, environmental monitoring data, corrective actions taken, and any deviations from standard operating procedures.
Fill out your cleanroom microbiology and sterility online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Cleanroom Microbiology And Sterility is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.


















