Get the free UK MHRA Publishes Guide to the New EU Medical Device ...
Show details
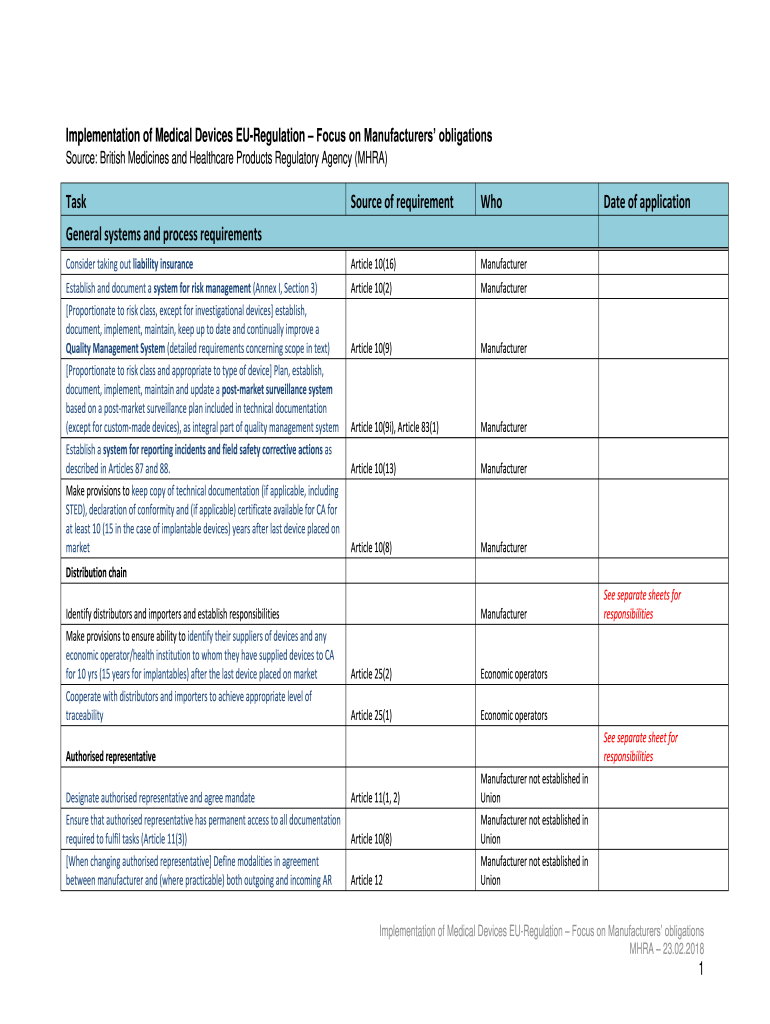
Implementation of Medical Devices Regulation Focus on Manufacturers obligations Source: British Medicines and Healthcare Products Regulatory Agency (MARA)Taskforce of requirementWhoConsider taking
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign uk mhra publishes guide

Edit your uk mhra publishes guide form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your uk mhra publishes guide form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit uk mhra publishes guide online
Follow the steps down below to take advantage of the professional PDF editor:
1
Log in. Click Start Free Trial and create a profile if necessary.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit uk mhra publishes guide. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Get your file. Select the name of your file in the docs list and choose your preferred exporting method. You can download it as a PDF, save it in another format, send it by email, or transfer it to the cloud.
Dealing with documents is always simple with pdfFiller. Try it right now
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out uk mhra publishes guide

How to fill out uk mhra publishes guide
01
To fill out the UK MHRA publishes guide, follow these steps:
02
Start by obtaining a copy of the guide from the official MHRA website.
03
Review the guide thoroughly to understand its purpose and scope.
04
Gather all the necessary information and documentation required for filling out the guide.
05
Begin by providing your organization's details, including name, address, and contact information.
06
Follow the guide's instructions to fill out each section accurately and completely.
07
Pay attention to any specific requirements or guidelines mentioned in the guide.
08
Use clear and concise language when providing information.
09
Double-check all the entered information for accuracy and completeness.
10
If there are any supporting documents or attachments required, ensure they are included.
11
Finally, submit the filled-out guide as per the specified submission process or deadline.
12
Note: It is recommended to seek professional advice or refer to the MHRA's guidance documents for any specific queries or concerns.
Who needs uk mhra publishes guide?
01
The UK MHRA publishes guide is mainly needed by organizations or individuals involved in activities regulated by the MHRA. This can include pharmaceutical companies, medical device manufacturers, clinical trial sponsors, wholesalers, distributors, and healthcare professionals. The guide provides important information and guidance on various regulatory requirements, processes, and obligations set by the MHRA. It helps ensure compliance with legal and safety standards, promoting the quality and safety of medicines, medical devices, and healthcare products in the UK.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I send uk mhra publishes guide for eSignature?
To distribute your uk mhra publishes guide, simply send it to others and receive the eSigned document back instantly. Post or email a PDF that you've notarized online. Doing so requires never leaving your account.
Can I create an eSignature for the uk mhra publishes guide in Gmail?
Upload, type, or draw a signature in Gmail with the help of pdfFiller’s add-on. pdfFiller enables you to eSign your uk mhra publishes guide and other documents right in your inbox. Register your account in order to save signed documents and your personal signatures.
How can I fill out uk mhra publishes guide on an iOS device?
Download and install the pdfFiller iOS app. Then, launch the app and log in or create an account to have access to all of the editing tools of the solution. Upload your uk mhra publishes guide from your device or cloud storage to open it, or input the document URL. After filling out all of the essential areas in the document and eSigning it (if necessary), you may save it or share it with others.
What is UK MHRA publishes guide?
The UK MHRA publishes guide provides essential information and guidance on the regulatory requirements for healthcare products in the UK, focusing on compliance with laws and safety standards.
Who is required to file UK MHRA publishes guide?
Manufacturers, importers, and distributors of medical devices, as well as other stakeholders involved in the supply chain of healthcare products in the UK, are required to file the UK MHRA publishes guide.
How to fill out UK MHRA publishes guide?
To fill out the UK MHRA publishes guide, stakeholders must follow the structured format provided in the guide, ensuring that all necessary information regarding product details, compliance status, and safety measures is accurately completed.
What is the purpose of UK MHRA publishes guide?
The purpose of the UK MHRA publishes guide is to ensure that healthcare products meet safety, quality, and regulatory standards, thereby protecting public health and aiding in informed decision-making for healthcare professionals and patients.
What information must be reported on UK MHRA publishes guide?
Information that must be reported includes product identification, manufacturer details, compliance status with relevant regulations, and any safety issues or adverse events associated with the product.
Fill out your uk mhra publishes guide online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Uk Mhra Publishes Guide is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















