Get the free MONOCLONAL ANTI-CD138 (C-TERMINAL) antibody produced in mouse
Show details
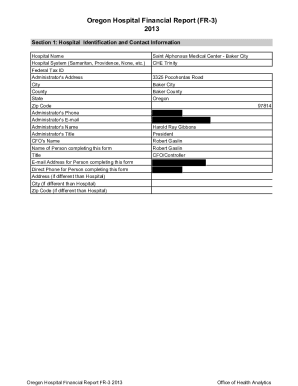
COABM401T7B6/template v2CERTIFICATE OF ANALYSIS
MONOCLONAL ANTIBODIESListeria SPP. Mouse monoclonal antibody
Code no.
Grade
Lot noBM401T7B6
Affinity PurifiedRECEIVER INFORMATION
Expiry Date
Storage
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign monoclonal anti-cd138 c-terminal antibody

Edit your monoclonal anti-cd138 c-terminal antibody form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your monoclonal anti-cd138 c-terminal antibody form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit monoclonal anti-cd138 c-terminal antibody online
To use our professional PDF editor, follow these steps:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit monoclonal anti-cd138 c-terminal antibody. Add and change text, add new objects, move pages, add watermarks and page numbers, and more. Then click Done when you're done editing and go to the Documents tab to merge or split the file. If you want to lock or unlock the file, click the lock or unlock button.
4
Save your file. Select it from your list of records. Then, move your cursor to the right toolbar and choose one of the exporting options. You can save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud, among other things.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out monoclonal anti-cd138 c-terminal antibody

How to fill out monoclonal anti-cd138 c-terminal antibody
01
Begin by preparing the monoclonal anti-CD138 C-terminal antibody. Follow the manufacturer's instructions for storage and handling.
02
Prepare the sample or tissue that you will be staining with the antibody. This may involve fixing the cells or performing antigen retrieval.
03
Dilute the antibody to the recommended concentration using an appropriate buffer or diluent. Follow the manufacturer's instructions for the correct dilution factor.
04
Apply the diluted antibody solution to the sample or tissue. Incubate for the recommended amount of time, typically 1-2 hours at room temperature or overnight at 4°C.
05
Wash the sample or tissue to remove any unbound antibody. Use an appropriate washing buffer and repeat this step multiple times.
06
Apply a secondary antibody if necessary, following the manufacturer's instructions for the specific secondary antibody used.
07
Incubate the sample or tissue with the secondary antibody for the recommended amount of time, typically 30 minutes to 1 hour.
08
Wash the sample or tissue again to remove any unbound secondary antibody.
09
Mount the sample or tissue on a microscope slide using an appropriate mounting medium.
10
Analyze the sample or tissue under a microscope or suitable imaging system, using the appropriate filters and imaging settings.
11
Interpret the results and document the findings.
Who needs monoclonal anti-cd138 c-terminal antibody?
01
Monoclonal anti-CD138 C-terminal antibody is needed by researchers and scientists who are studying CD138, a surface glycoprotein found on plasma cells.
02
It may be used in various research applications, including immunohistochemistry, immunocytochemistry, and flow cytometry.
03
This antibody is particularly useful in the study of multiple myeloma, a type of cancer that involves abnormal plasma cells.
04
It can help researchers identify and characterize CD138-positive plasma cells in tissue or cell samples.
05
Therefore, individuals involved in the field of cancer research, immunology, or cell biology may need monoclonal anti-CD138 C-terminal antibody.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I modify my monoclonal anti-cd138 c-terminal antibody in Gmail?
pdfFiller’s add-on for Gmail enables you to create, edit, fill out and eSign your monoclonal anti-cd138 c-terminal antibody and any other documents you receive right in your inbox. Visit Google Workspace Marketplace and install pdfFiller for Gmail. Get rid of time-consuming steps and manage your documents and eSignatures effortlessly.
Can I create an eSignature for the monoclonal anti-cd138 c-terminal antibody in Gmail?
With pdfFiller's add-on, you may upload, type, or draw a signature in Gmail. You can eSign your monoclonal anti-cd138 c-terminal antibody and other papers directly in your mailbox with pdfFiller. To preserve signed papers and your personal signatures, create an account.
How do I edit monoclonal anti-cd138 c-terminal antibody on an iOS device?
Use the pdfFiller app for iOS to make, edit, and share monoclonal anti-cd138 c-terminal antibody from your phone. Apple's store will have it up and running in no time. It's possible to get a free trial and choose a subscription plan that fits your needs.
What is monoclonal anti-cd138 c-terminal antibody?
Monoclonal anti-CD138 C-terminal antibody is a specific type of antibody that targets the CD138 protein, often used in research and clinical settings for the identification and isolation of plasma cells in various diseases, particularly multiple myeloma.
Who is required to file monoclonal anti-cd138 c-terminal antibody?
Researchers, healthcare professionals, and institutions utilizing the monoclonal anti-CD138 C-terminal antibody for experimental or therapeutic purposes may be required to file documentation regarding its use, depending on regional regulations.
How to fill out monoclonal anti-cd138 c-terminal antibody?
Filling out documentation for monoclonal anti-CD138 C-terminal antibody typically involves providing specific details about the antibody, including its source, intended use, dosage, and safety data, as well as compliance with applicable regulations.
What is the purpose of monoclonal anti-cd138 c-terminal antibody?
The primary purpose of monoclonal anti-CD138 C-terminal antibody is to specifically bind to CD138, facilitating the detection and study of plasma cells in laboratory research and clinical diagnostics.
What information must be reported on monoclonal anti-cd138 c-terminal antibody?
Information that must be reported typically includes the antibody's characterization, source, batch number, intended application, experimental data, and compliance with safety and regulatory standards.
Fill out your monoclonal anti-cd138 c-terminal antibody online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Monoclonal Anti-cd138 C-Terminal Antibody is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.