Get the free Solubility EquilibriabApplicationb of already known Ideas - oakton
Show details
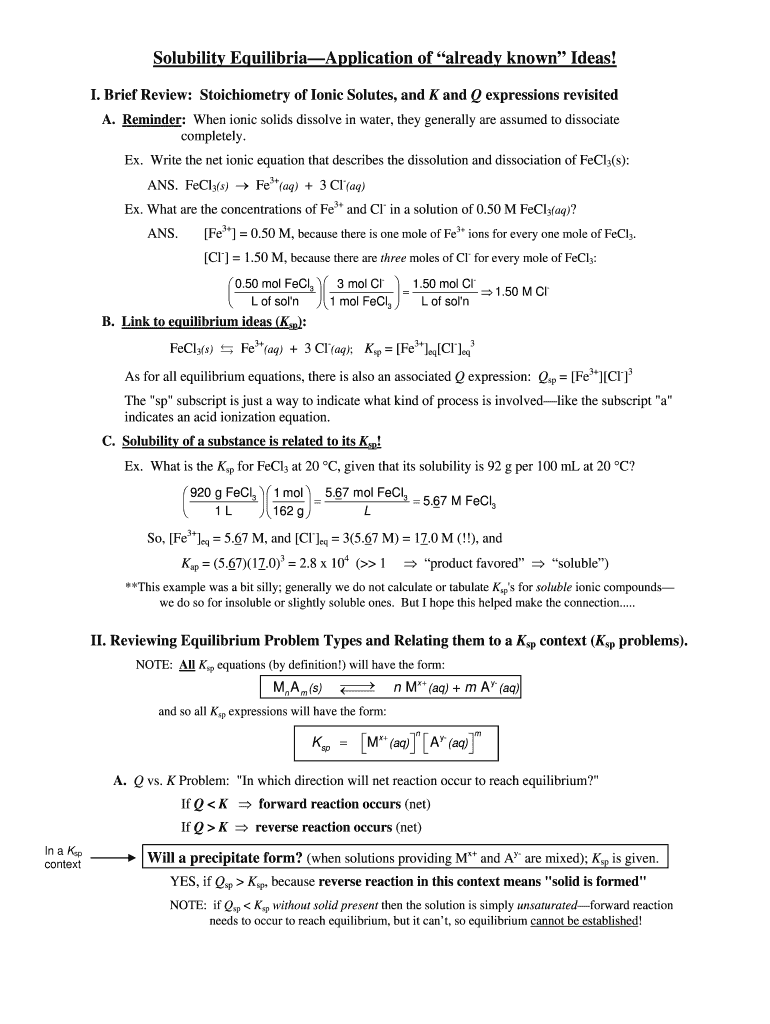
Solubility EquilibriaApplication of already known Ideas! I. Brief Review: Stoichiometry of Ionic Solutes, and K and Q expressions revisited A. Reminder: When ionic solids dissolve in water, they generally
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign solubility equilibriabapplicationb of already

Edit your solubility equilibriabapplicationb of already form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your solubility equilibriabapplicationb of already form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit solubility equilibriabapplicationb of already online
Use the instructions below to start using our professional PDF editor:
1
Create an account. Begin by choosing Start Free Trial and, if you are a new user, establish a profile.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit solubility equilibriabapplicationb of already. Add and change text, add new objects, move pages, add watermarks and page numbers, and more. Then click Done when you're done editing and go to the Documents tab to merge or split the file. If you want to lock or unlock the file, click the lock or unlock button.
4
Get your file. When you find your file in the docs list, click on its name and choose how you want to save it. To get the PDF, you can save it, send an email with it, or move it to the cloud.
The use of pdfFiller makes dealing with documents straightforward. Try it now!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out solubility equilibriabapplicationb of already

How to fill out solubility equilibrium application form:
01
Start by carefully reading the instructions provided on the form. This will give you an understanding of the required information and the format in which it needs to be filled out.
02
Begin by providing your personal details, such as your name, contact information, and any other requested identification information.
03
In the form, you will likely be required to mention the solubility equilibrium you are referring to. State the specific solubility equilibrium you are interested in studying or researching.
04
Next, you may be asked to outline your objectives or reasons for studying the solubility equilibrium. Explain why you are interested in this specific area and what you hope to achieve by filling out the application.
05
The form may also require you to provide details about your educational background and any relevant experience or coursework you have completed in the field of solubility equilibrium.
06
If applicable, include any research or project proposals related to the solubility equilibrium. Describe the experiments you plan to conduct, the methodologies you will use, and the expected outcomes.
07
In order to support your application, you may be asked to attach additional documents, such as your resume, academic transcripts, or letters of recommendation. Ensure that you have all the necessary documents ready before filling out the form.
08
Before submitting the form, review all the information you have provided to ensure accuracy and completeness. Make any necessary corrections or additions.
09
Finally, sign and date the form, if required. Follow any additional instructions provided in order to submit the application successfully.
Who needs solubility equilibrium application form:
01
Students studying chemistry or related scientific fields who are interested in researching or conducting experiments on solubility equilibrium.
02
Researchers or scientists working in academic or industrial settings who require detailed information and understanding of solubility equilibrium in their field of study.
03
Individuals or organizations seeking funding or grants for research projects related to solubility equilibrium may need to fill out this application form to access the necessary resources.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I manage my solubility equilibriabapplicationb of already directly from Gmail?
pdfFiller’s add-on for Gmail enables you to create, edit, fill out and eSign your solubility equilibriabapplicationb of already and any other documents you receive right in your inbox. Visit Google Workspace Marketplace and install pdfFiller for Gmail. Get rid of time-consuming steps and manage your documents and eSignatures effortlessly.
How can I send solubility equilibriabapplicationb of already to be eSigned by others?
To distribute your solubility equilibriabapplicationb of already, simply send it to others and receive the eSigned document back instantly. Post or email a PDF that you've notarized online. Doing so requires never leaving your account.
How do I edit solubility equilibriabapplicationb of already on an iOS device?
Use the pdfFiller mobile app to create, edit, and share solubility equilibriabapplicationb of already from your iOS device. Install it from the Apple Store in seconds. You can benefit from a free trial and choose a subscription that suits your needs.
What is solubility equilibrium of already?
Solubility equilibrium refers to the state where the rate of dissolution of a solute is equal to the rate of precipitation of that solute in a solution.
Who is required to file solubility equilibrium of already?
Solubility equilibrium is a concept in chemistry and is not typically something that requires filing.
How to fill out solubility equilibrium of already?
To calculate solubility equilibrium, one must set up the equilibrium expression based on the balanced chemical equation for the dissolution or precipitation reaction.
What is the purpose of solubility equilibrium of already?
The purpose of understanding solubility equilibrium is to predict whether a solute will dissolve in a solvent or precipitate out of the solution.
What information must be reported on solubility equilibrium of already?
Information such as the initial concentrations of the solute and solvent, the equilibrium concentrations of the solute and solvent, and the equilibrium constant must be reported.
Fill out your solubility equilibriabapplicationb of already online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Solubility Equilibriabapplicationb Of Already is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.

















