Get the free Non-clinical studies in the process of new drug ...
Show details
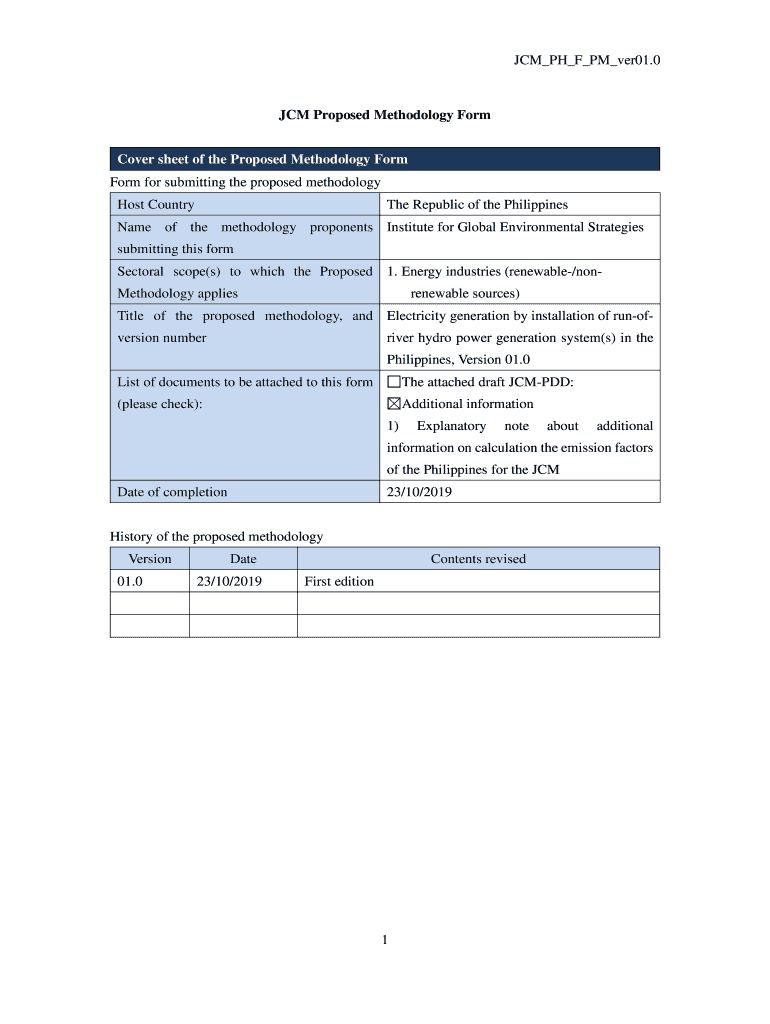
JCM_PH_F_PM_ver01.0JCM Proposed Methodology Form
Cover sheet of the Proposed Methodology Form for submitting the proposed methodology
Host Country Republic of the PhilippinesName of the methodology
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign non-clinical studies in form

Edit your non-clinical studies in form form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your non-clinical studies in form form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit non-clinical studies in form online
Follow the guidelines below to benefit from a competent PDF editor:
1
Check your account. If you don't have a profile yet, click Start Free Trial and sign up for one.
2
Upload a file. Select Add New on your Dashboard and upload a file from your device or import it from the cloud, online, or internal mail. Then click Edit.
3
Edit non-clinical studies in form. Rearrange and rotate pages, add new and changed texts, add new objects, and use other useful tools. When you're done, click Done. You can use the Documents tab to merge, split, lock, or unlock your files.
4
Get your file. When you find your file in the docs list, click on its name and choose how you want to save it. To get the PDF, you can save it, send an email with it, or move it to the cloud.
pdfFiller makes working with documents easier than you could ever imagine. Create an account to find out for yourself how it works!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out non-clinical studies in form

How to fill out non-clinical studies in form
01
To fill out non-clinical studies in form, follow these steps:
02
Start by gathering all the necessary information and documents related to the non-clinical studies.
03
Fill out the form with accurate and detailed information about the non-clinical studies.
04
Provide a clear description of the objectives, methodology, and results of the non-clinical studies.
05
Include any supporting data, graphs, or charts that illustrate the findings of the non-clinical studies.
06
Ensure all information is complete and organized in a logical manner.
07
Review the form for any errors or missing information.
08
Double-check that all required fields are filled out correctly.
09
Submit the form according to the specified guidelines and deadlines.
10
Keep a copy of the filled-out form and any accompanying documents for your records.
Who needs non-clinical studies in form?
01
Non-clinical studies in form are needed by individuals or organizations involved in research and development of pharmaceuticals, biologics, medical devices, or any other product that requires safety and efficacy testing.
02
These studies provide crucial information about the potential risks, benefits, and effects of the product on living organisms.
03
Regulatory authorities, such as the FDA (Food and Drug Administration) or other health agencies, often require non-clinical studies to ensure the safety and effectiveness of the product before it can be approved for clinical trials or commercialization.
04
Pharmaceutical companies, research institutions, and academic laboratories also conduct non-clinical studies to gather data for scientific publications, patents, or internal decision-making processes.
05
In summary, anyone involved in the development and evaluation of products that impact human or animal health may need to fill out non-clinical studies in form.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I execute non-clinical studies in form online?
pdfFiller has made it easy to fill out and sign non-clinical studies in form. You can use the solution to change and move PDF content, add fields that can be filled in, and sign the document electronically. Start a free trial of pdfFiller, the best tool for editing and filling in documents.
How do I edit non-clinical studies in form online?
With pdfFiller, the editing process is straightforward. Open your non-clinical studies in form in the editor, which is highly intuitive and easy to use. There, you’ll be able to blackout, redact, type, and erase text, add images, draw arrows and lines, place sticky notes and text boxes, and much more.
How can I edit non-clinical studies in form on a smartphone?
The easiest way to edit documents on a mobile device is using pdfFiller’s mobile-native apps for iOS and Android. You can download those from the Apple Store and Google Play, respectively. You can learn more about the apps here. Install and log in to the application to start editing non-clinical studies in form.
What is non-clinical studies in form?
Non-clinical studies in form refer to the documentation and data collected from studies conducted outside of a clinical setting.
Who is required to file non-clinical studies in form?
Individuals or organizations conducting non-clinical studies are required to file the necessary forms.
How to fill out non-clinical studies in form?
Non-clinical studies forms can be filled out electronically or manually, providing detailed information about the study objectives, methods, and findings.
What is the purpose of non-clinical studies in form?
The purpose of non-clinical studies in form is to ensure transparency and accountability in research, as well as to provide valuable information for regulatory review.
What information must be reported on non-clinical studies in form?
Non-clinical studies forms typically require information such as study design, animal models used, dosages administered, and results obtained.
Fill out your non-clinical studies in form online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Non-Clinical Studies In Form is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.


















