Get the free Protection of Human Subjects: Assurance Identification/IRB ...
Show details
OMB No. 09900263
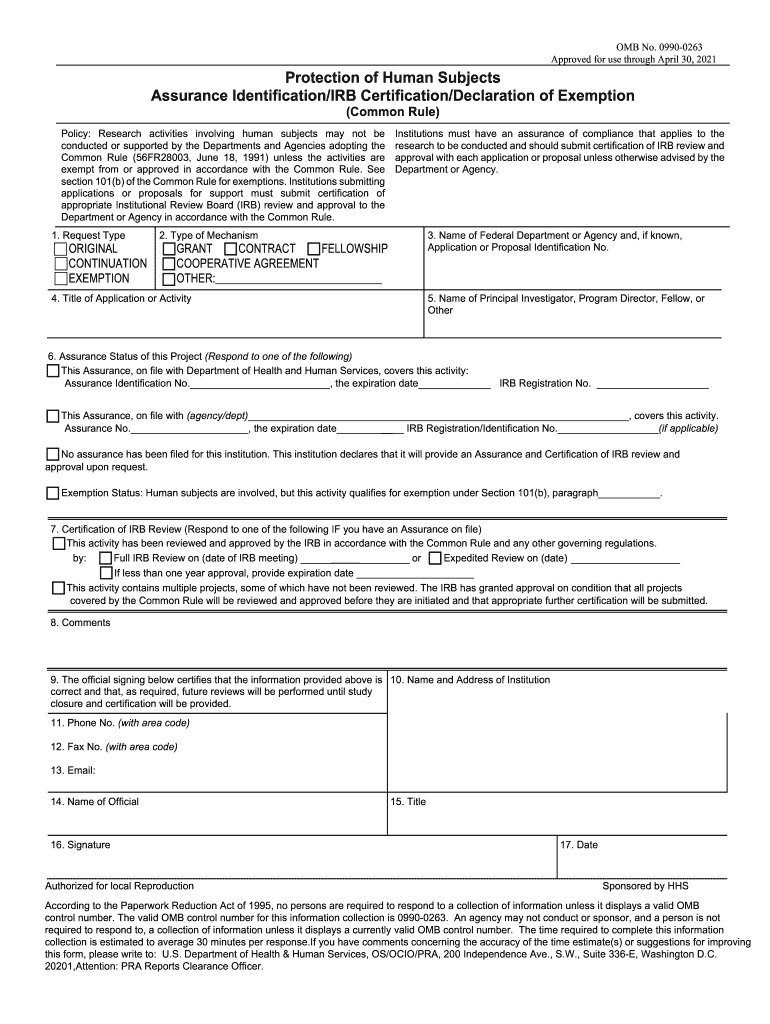
Approved for use through April 30, 2021Protection of Human Subjects
Assurance Identification/IRB Certification/Declaration of Exemption(Common Rule)Policy: Research activities involving
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign protection of human subjects

Edit your protection of human subjects form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your protection of human subjects form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit protection of human subjects online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Upload a document. Select Add New on your Dashboard and transfer a file into the system in one of the following ways: by uploading it from your device or importing from the cloud, web, or internal mail. Then, click Start editing.
3
Edit protection of human subjects. Replace text, adding objects, rearranging pages, and more. Then select the Documents tab to combine, divide, lock or unlock the file.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
It's easier to work with documents with pdfFiller than you could have ever thought. Sign up for a free account to view.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out protection of human subjects

How to fill out protection of human subjects
01
Review the regulations and guidelines provided by the governing body for the protection of human subjects. Understand the requirements and responsibilities involved.
02
Determine if the proposed research involves human subjects. It is important to define what constitutes a human subject and ensure that the research falls under the purview of the regulations.
03
Assess the potential risks and benefits associated with the research. This includes evaluating the nature of the research, the population being studied, and any potential harm or discomfort that may arise.
04
Develop a clear and comprehensive informed consent process. This involves providing potential subjects with all the necessary information about the research, its purpose, risks, benefits, and any alternatives. Make sure that informed consent is obtained in a voluntary and documented manner.
05
Design the research study and methodology with safeguards in place to protect the subjects. This may include measures such as anonymity, confidentiality, privacy protection, and minimizing potential harm or discomfort.
06
Seek ethical review and approval from a relevant Institutional Review Board (IRB) or ethics committee. Submit all necessary documentation and protocols for review before initiating the research.
07
Implement the approved research study while adhering to the guidelines, regulations, and ethical considerations. Continuously monitor the progress of the study and promptly address any ethical concerns or issues that arise.
08
Report the findings of the research study in a transparent and responsible manner. Ensure that any potential risks, harm, or unexpected outcomes are appropriately communicated and addressed.
09
Ensure ongoing compliance with the regulations and guidelines for the protection of human subjects throughout the entire research process. This includes periodic review, reassessment, and modification of protocols as needed.
Who needs protection of human subjects?
01
Researchers and professionals conducting scientific studies involving human participants or subjects.
02
Academic institutions and research organizations that oversee or sponsor research projects.
03
Ethics committees or Institutional Review Boards (IRBs) responsible for reviewing and approving research protocols.
04
Funding agencies or organizations providing financial support for research involving human subjects.
05
Government regulatory bodies and policy-makers responsible for establishing and enforcing regulations and guidelines for human subjects protection.
06
Community and public interest groups concerned with the ethical conduct of research involving human subjects.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
Can I create an electronic signature for the protection of human subjects in Chrome?
You can. With pdfFiller, you get a strong e-signature solution built right into your Chrome browser. Using our addon, you may produce a legally enforceable eSignature by typing, sketching, or photographing it. Choose your preferred method and eSign in minutes.
How do I fill out the protection of human subjects form on my smartphone?
You can easily create and fill out legal forms with the help of the pdfFiller mobile app. Complete and sign protection of human subjects and other documents on your mobile device using the application. Visit pdfFiller’s webpage to learn more about the functionalities of the PDF editor.
How do I edit protection of human subjects on an iOS device?
Create, modify, and share protection of human subjects using the pdfFiller iOS app. Easy to install from the Apple Store. You may sign up for a free trial and then purchase a membership.
What is protection of human subjects?
Protection of human subjects refers to the ethical and legal standards that must be followed in research involving human participants to ensure their rights, safety, and well-being are protected.
Who is required to file protection of human subjects?
Researchers conducting studies involving human participants are required to file protection of human subjects.
How to fill out protection of human subjects?
To fill out protection of human subjects, researchers need to provide detailed information about the study protocol, risks and benefits to participants, informed consent process, and plans for protecting participant confidentiality and data.
What is the purpose of protection of human subjects?
The purpose of protection of human subjects is to safeguard the rights, safety, and well-being of human participants in research studies.
What information must be reported on protection of human subjects?
Information that must be reported on protection of human subjects includes study details, risks and benefits assessment, informed consent process, participant confidentiality measures, and data handling procedures.
Fill out your protection of human subjects online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Protection Of Human Subjects is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















