Get the free Development of Diagnostic Instruments for Research Study Skills in ...
Show details
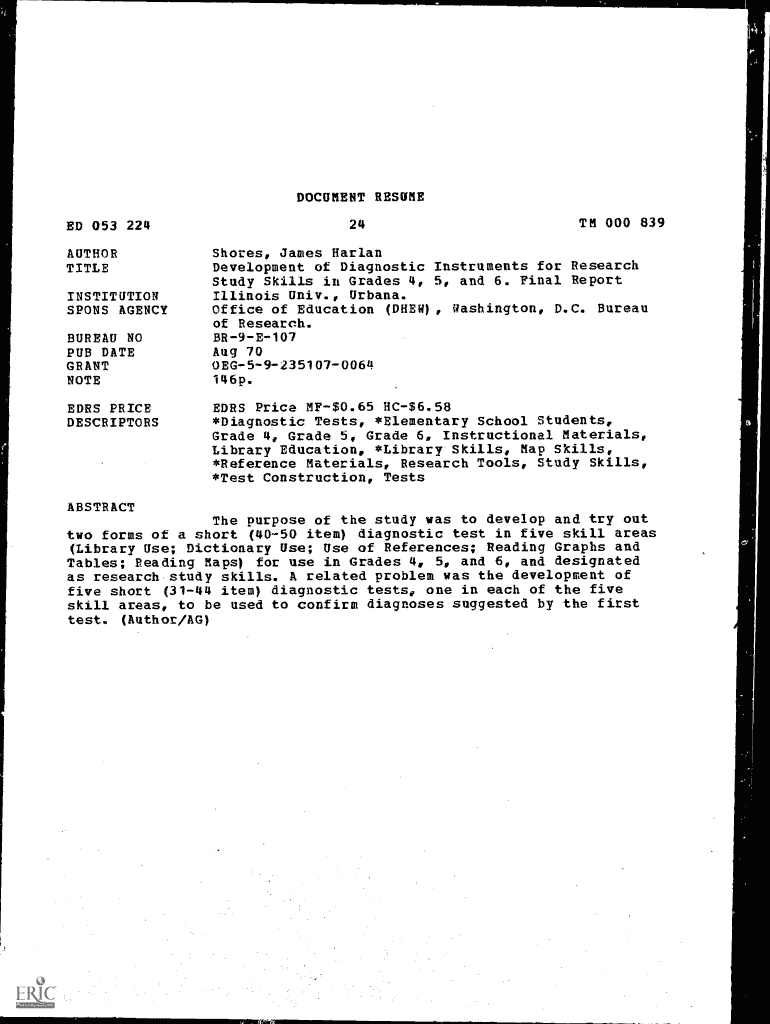
DOCUMENT RESUME
24ED 053 224AUTHOR
TITLE
INSTITUTION
SONS AGENCY
BUREAU NO
PUB DATE
GRANT
OTHERS PRICE
DESCRIPTOR STM 000 839Shores, James Harlan
Development of Diagnostic Instruments for Research
Study
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign development of diagnostic instruments

Edit your development of diagnostic instruments form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your development of diagnostic instruments form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing development of diagnostic instruments online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Log in to your account. Start Free Trial and register a profile if you don't have one.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit development of diagnostic instruments. Replace text, adding objects, rearranging pages, and more. Then select the Documents tab to combine, divide, lock or unlock the file.
4
Save your file. Select it in the list of your records. Then, move the cursor to the right toolbar and choose one of the available exporting methods: save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud.
pdfFiller makes working with documents easier than you could ever imagine. Try it for yourself by creating an account!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out development of diagnostic instruments

How to fill out development of diagnostic instruments
01
Start by understanding the purpose and requirements of the diagnostic instrument development project.
02
Conduct thorough research on existing diagnostic instruments in the market and identify areas for improvement or innovation.
03
Define the key parameters and features that your diagnostic instrument should have.
04
Design a detailed development plan, including timelines, resources required, and budget.
05
Gather a team of experts in relevant fields such as engineering, software development, and data analysis.
06
Develop a prototype of the diagnostic instrument using appropriate technologies and methodologies.
07
Test the prototype extensively in a controlled environment to ensure accuracy and reliability of results.
08
Continuously iterate and improve the prototype based on user feedback and test results.
09
Obtain necessary regulatory approvals and certifications for the diagnostic instrument.
10
Prepare for mass production and distribution of the final diagnostic instrument.
11
Provide adequate training and support for users of the diagnostic instrument.
12
Monitor and gather feedback on the performance of the diagnostic instrument in real-world scenarios, and make necessary updates or improvements.
13
Stay updated with the latest advancements in diagnostic technology and continually strive to enhance your diagnostic instrument.
Who needs development of diagnostic instruments?
01
Medical professionals and healthcare organizations that require reliable and accurate diagnostic tools for patient care and treatment.
02
Research institutions and laboratories that perform diagnostic testing and require advanced instruments for their studies.
03
Biotechnology and pharmaceutical companies that develop and produce diagnostic products for various diseases.
04
Medical device manufacturers who aim to expand their product portfolio and meet the growing demand for diagnostic instruments.
05
Government bodies and regulatory agencies that oversee the quality and safety of diagnostic instruments in the market.
06
Patients who benefit from timely and accurate diagnosis through the use of advanced diagnostic instruments.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I send development of diagnostic instruments to be eSigned by others?
When your development of diagnostic instruments is finished, send it to recipients securely and gather eSignatures with pdfFiller. You may email, text, fax, mail, or notarize a PDF straight from your account. Create an account today to test it.
Where do I find development of diagnostic instruments?
The premium version of pdfFiller gives you access to a huge library of fillable forms (more than 25 million fillable templates). You can download, fill out, print, and sign them all. State-specific development of diagnostic instruments and other forms will be easy to find in the library. Find the template you need and use advanced editing tools to make it your own.
How do I edit development of diagnostic instruments on an Android device?
You can edit, sign, and distribute development of diagnostic instruments on your mobile device from anywhere using the pdfFiller mobile app for Android; all you need is an internet connection. Download the app and begin streamlining your document workflow from anywhere.
What is development of diagnostic instruments?
Development of diagnostic instruments refers to the process of designing, creating, and improving tools used for diagnosing medical conditions.
Who is required to file development of diagnostic instruments?
Manufacturers, researchers, and developers of diagnostic instruments are required to file development reports.
How to fill out development of diagnostic instruments?
Development reports for diagnostic instruments should include detailed information on the design, testing, and validation of the instrument.
What is the purpose of development of diagnostic instruments?
The purpose of development reports for diagnostic instruments is to ensure that the instruments are accurate, reliable, and safe for use in medical settings.
What information must be reported on development of diagnostic instruments?
Information such as design specifications, testing protocols, validation results, and any potential risks or limitations must be reported on development reports.
Fill out your development of diagnostic instruments online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Development Of Diagnostic Instruments is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.




















