Get the free RELEASE OF DRUG AND ALCOHOL ABUSE RECORDS
Show details
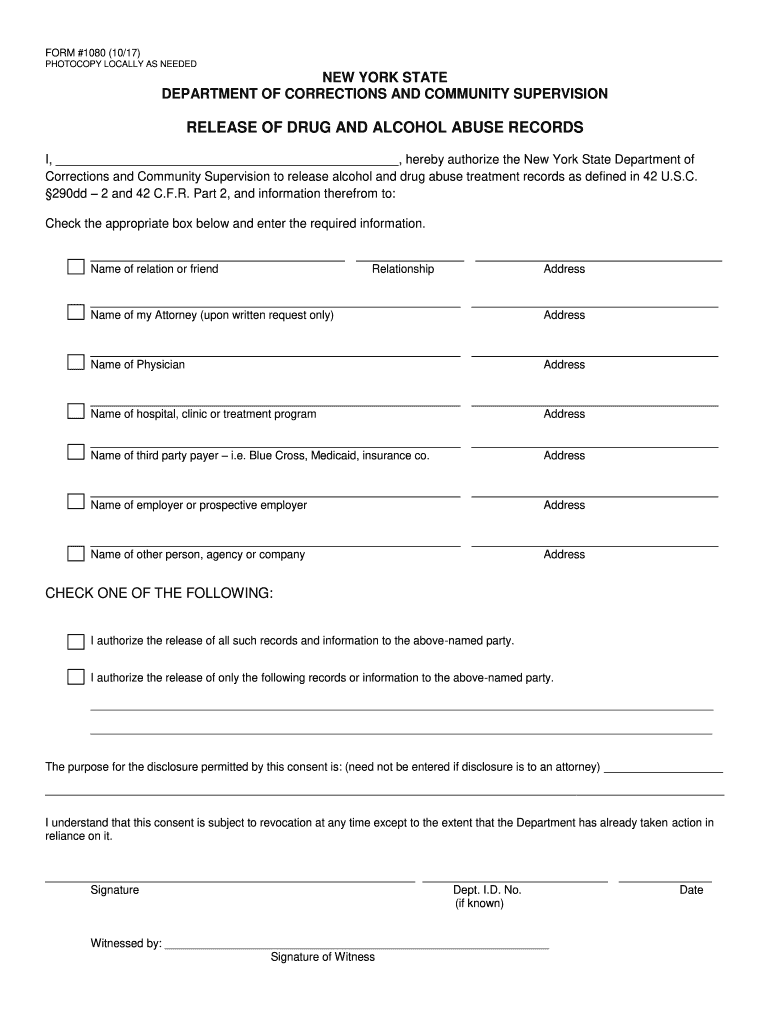
FORM #1080 (10/17) PHOTOCOPY LOCALLY AS NEEDED YORK STATE DEPARTMENT OF CORRECTIONS AND COMMUNITY SUPERVISIONRELEASE OF DRUG AND ALCOHOL ABUSE RECORDS I, hereby authorize the New York State Department
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign release of drug and

Edit your release of drug and form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your release of drug and form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit release of drug and online
Follow the guidelines below to benefit from a competent PDF editor:
1
Create an account. Begin by choosing Start Free Trial and, if you are a new user, establish a profile.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit release of drug and. Add and replace text, insert new objects, rearrange pages, add watermarks and page numbers, and more. Click Done when you are finished editing and go to the Documents tab to merge, split, lock or unlock the file.
4
Save your file. Choose it from the list of records. Then, shift the pointer to the right toolbar and select one of the several exporting methods: save it in multiple formats, download it as a PDF, email it, or save it to the cloud.
With pdfFiller, it's always easy to work with documents. Check it out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out release of drug and

How to fill out release of drug and
01
Step 1: Start by gathering all the necessary information about the drug that needs to be released. This includes the drug name, dosage, expiry date, and any precautions or side effects associated with it.
02
Step 2: Check the requirements of the regulatory authorities or governing bodies for releasing a drug. Each country may have different procedures and documentation needed.
03
Step 3: Prepare all the required documents such as the release application form, product information leaflet, safety data sheet, and batch manufacturing records.
04
Step 4: Review and ensure all the documents are accurate, complete, and compliant with the regulatory guidelines.
05
Step 5: Submit the release application along with the supporting documents to the appropriate regulatory authority.
06
Step 6: Wait for the regulatory authority's review and approval process. This may involve inspections, testing of samples, and evaluation of the documentation.
07
Step 7: If the drug passes all the necessary checks, it will be released and can be distributed for use.
08
Step 8: Keep records of the release process and any relevant communication with the regulatory authority for future reference or audits.
Who needs release of drug and?
01
Pharmaceutical manufacturers who have developed a new drug or modified an existing drug and intend to release it for commercial use.
02
Hospitals, clinics, and healthcare facilities that need to ensure the safety and effectiveness of the drugs they distribute to patients.
03
Pharmacists who dispense medications to patients and need to ensure the quality and authenticity of the drugs they provide.
04
Regulatory authorities or governing bodies responsible for overseeing the release and distribution of drugs to protect public health.
05
Researchers and scientists involved in clinical trials or drug development who require proper documentation and verification of drug release.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I make changes in release of drug and?
pdfFiller not only allows you to edit the content of your files but fully rearrange them by changing the number and sequence of pages. Upload your release of drug and to the editor and make any required adjustments in a couple of clicks. The editor enables you to blackout, type, and erase text in PDFs, add images, sticky notes and text boxes, and much more.
Can I create an electronic signature for the release of drug and in Chrome?
Yes. You can use pdfFiller to sign documents and use all of the features of the PDF editor in one place if you add this solution to Chrome. In order to use the extension, you can draw or write an electronic signature. You can also upload a picture of your handwritten signature. There is no need to worry about how long it takes to sign your release of drug and.
How do I fill out release of drug and using my mobile device?
You can quickly make and fill out legal forms with the help of the pdfFiller app on your phone. Complete and sign release of drug and and other documents on your mobile device using the application. If you want to learn more about how the PDF editor works, go to pdfFiller.com.
What is release of drug and?
Release of drug refers to the official process by which the regulatory authority permits a drug to be made available for public use after it has met necessary safety, efficacy, and quality standards.
Who is required to file release of drug and?
Pharmaceutical companies and manufacturers are required to file for the release of a drug, typically through regulatory agencies like the FDA.
How to fill out release of drug and?
To fill out the release of drug form, one must provide detailed information about the drug, including its formulation, testing results, manufacturing practices, and intended use, along with any other required documentation as specified by the regulatory authority.
What is the purpose of release of drug and?
The purpose of the release of drug is to ensure that the drug is safe for public consumption, effective for its intended use, and manufactured according to guidelines that ensure its quality.
What information must be reported on release of drug and?
The information that must be reported includes the drug's active and inactive ingredients, dosage form, administration route, labeling, evidence of clinical trials, and manufacturing details.
Fill out your release of drug and online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Release Of Drug And is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.




















