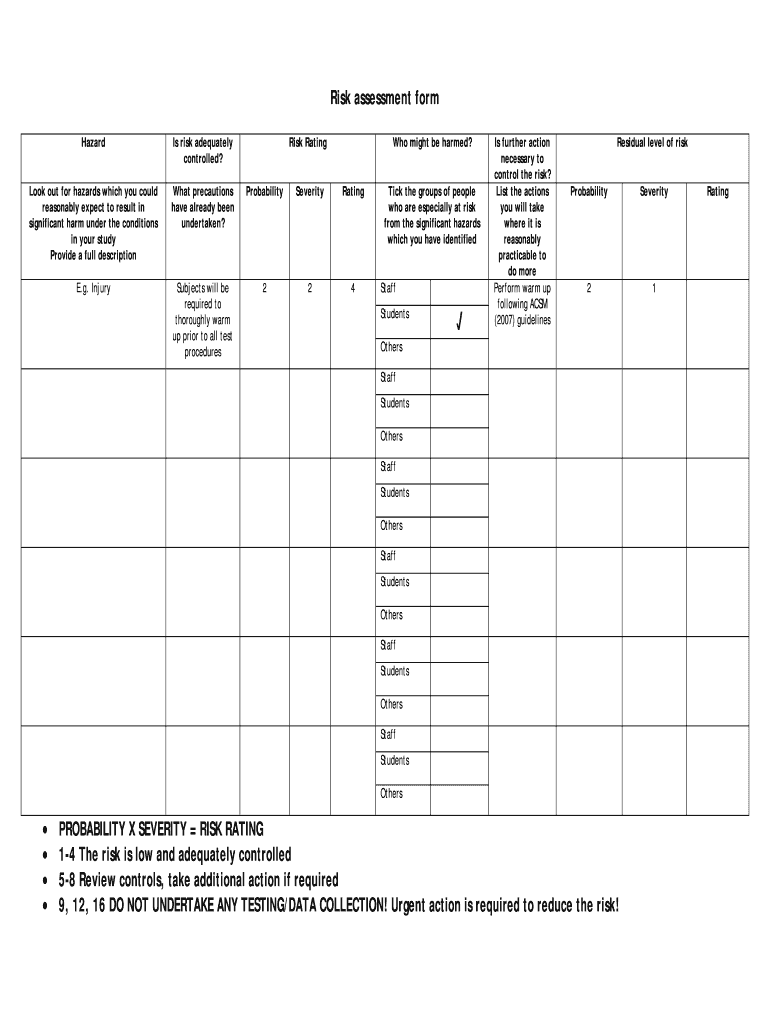
Get the free Ethical approval - Risk assessment form - Portal
Show details
The key themes identified were: conduct of assessment boards, ... Approve 'real- world×39; learning as the theme for further detailed analysis and .... FUSE had made good progress in rolling out
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign ethical approval - risk

Edit your ethical approval - risk form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your ethical approval - risk form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit ethical approval - risk online
In order to make advantage of the professional PDF editor, follow these steps below:
1
Create an account. Begin by choosing Start Free Trial and, if you are a new user, establish a profile.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit ethical approval - risk. Replace text, adding objects, rearranging pages, and more. Then select the Documents tab to combine, divide, lock or unlock the file.
4
Get your file. Select the name of your file in the docs list and choose your preferred exporting method. You can download it as a PDF, save it in another format, send it by email, or transfer it to the cloud.
Dealing with documents is simple using pdfFiller.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out ethical approval - risk

How to fill out ethical approval - risk:
01
Start by carefully reading and understanding the guidelines and requirements for ethical approval - risk. This will help ensure that you provide all the necessary information and meet the necessary criteria.
02
Gather all the relevant information and data related to the potential risks associated with your project or research. This may include information about the participants, potential harm or discomfort, and any measures you plan to take to minimize or mitigate these risks.
03
Clearly articulate the potential risks in your application. Provide detailed descriptions of the risks involved in your project and explain how you plan to address them. This could include steps you will take to mitigate risks, obtain informed consent from participants, or ensure confidentiality and privacy.
04
Consider involving an ethics committee or seeking input from colleagues or experts in the field to ensure that you have adequately addressed all potential risks in your application. They can provide valuable insights and suggestions for improvement.
05
Be honest and transparent in your application. It is important to accurately represent the potential risks associated with your project and not downplay or omit any important information. This will help ensure the ethical review process is thorough and effective.
Who needs ethical approval - risk?
01
Researchers conducting studies or projects that involve human participants are typically required to obtain ethical approval - risk. This includes both scientific research and other types of studies, such as social science research or biomedical research.
02
Academic institutions, funding agencies, and regulatory bodies often require ethical approval - risk to ensure that research is conducted ethically and protects the rights and well-being of the participants involved.
03
Ethical approval - risk is also necessary for projects or studies that involve potentially vulnerable populations, such as children, prisoners, or individuals with cognitive impairments. Special care and attention must be given to ensure their rights and welfare are protected.
04
In some cases, ethical approval - risk may also be required for projects or studies that involve the collection and analysis of sensitive or personal data, even if human participants are not directly involved. This ensures that privacy and confidentiality are maintained and that the data is used responsibly and ethically.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I send ethical approval - risk for eSignature?
When your ethical approval - risk is finished, send it to recipients securely and gather eSignatures with pdfFiller. You may email, text, fax, mail, or notarize a PDF straight from your account. Create an account today to test it.
How do I edit ethical approval - risk online?
pdfFiller not only lets you change the content of your files, but you can also change the number and order of pages. Upload your ethical approval - risk to the editor and make any changes in a few clicks. The editor lets you black out, type, and erase text in PDFs. You can also add images, sticky notes, and text boxes, as well as many other things.
How do I complete ethical approval - risk on an Android device?
Use the pdfFiller mobile app to complete your ethical approval - risk on an Android device. The application makes it possible to perform all needed document management manipulations, like adding, editing, and removing text, signing, annotating, and more. All you need is your smartphone and an internet connection.
What is ethical approval - risk?
Ethical approval - risk is the process of assessing the potential risks and ethical considerations associated with a research study or project.
Who is required to file ethical approval - risk?
Researchers, scientists, or institutions conducting research studies or projects that involve human participants are required to file ethical approval - risk.
How to fill out ethical approval - risk?
Ethical approval - risk forms typically require detailed information about the study design, risks to participants, ethical considerations, and consent procedures.
What is the purpose of ethical approval - risk?
The purpose of ethical approval - risk is to protect the rights and well-being of research participants, ensure ethical conduct in research, and mitigate potential risks.
What information must be reported on ethical approval - risk?
Information such as study objectives, methodology, potential risks to participants, ethical considerations, consent procedures, and data management plans must be reported on ethical approval - risk forms.
Fill out your ethical approval - risk online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Ethical Approval - Risk is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















