Get the free CLIA Certification # 36D0655844 - odh ohio
Show details
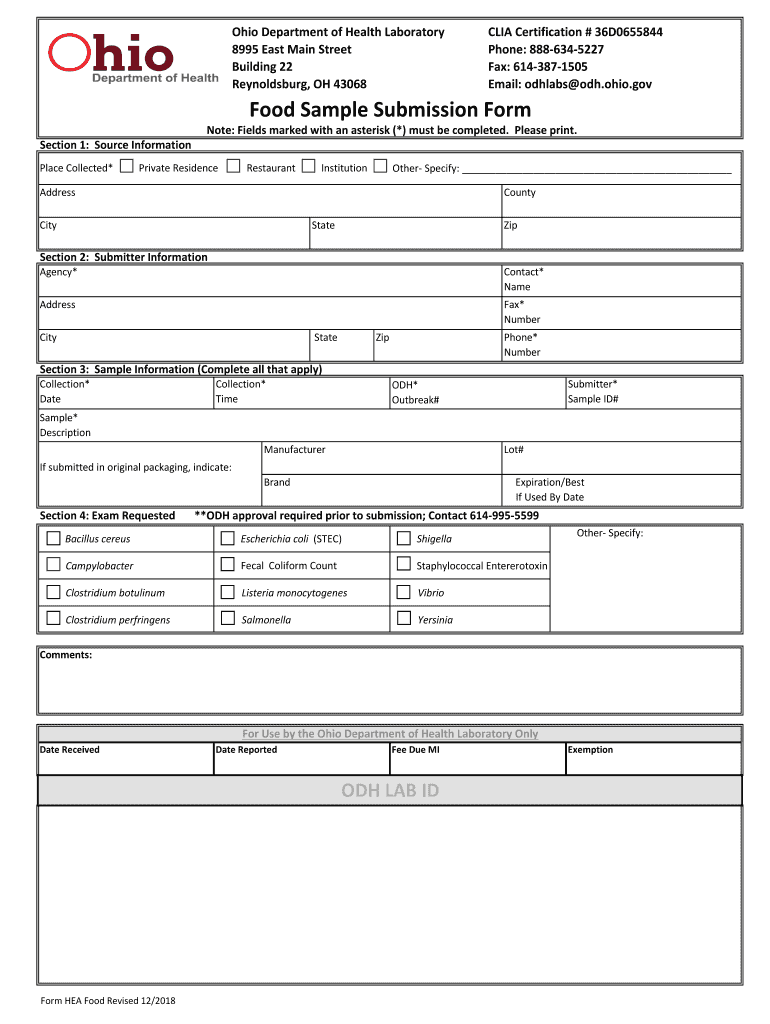
Ohio Department of Health Laboratory 8995 East Main Street Building 22 Reynoldsburg, OH 43068CLIA Certification # 36D0655844 Phone: 8886345227 Fax: 6143871505 Email: dollars odd. Ohio.godhood Sample
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign clia certification 36d0655844

Edit your clia certification 36d0655844 form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your clia certification 36d0655844 form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing clia certification 36d0655844 online
To use our professional PDF editor, follow these steps:
1
Log in. Click Start Free Trial and create a profile if necessary.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit clia certification 36d0655844. Replace text, adding objects, rearranging pages, and more. Then select the Documents tab to combine, divide, lock or unlock the file.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
pdfFiller makes dealing with documents a breeze. Create an account to find out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out clia certification 36d0655844

How to fill out clia certification 36d0655844
01
To fill out the CLIA certification form 36d0655844, follow these steps:
02
Start by reviewing the instructions provided on the form. It will give you an overview of the information required and any specific guidelines.
03
Gather all the necessary documents and supporting evidence needed to complete the certification. This may include proof of qualifications, training certificates, and relevant experience.
04
Fill in the personal information section accurately. Provide your full name, contact details, and any identifiers required.
05
Answer all the questions in the form truthfully and to the best of your knowledge. Double-check your responses for accuracy before submitting.
06
If there are any additional sections or attachments required, make sure to include them as specified on the form.
07
Review the completed form to ensure all sections are filled out correctly and there are no errors or omissions.
08
Submit the filled-out CLIA certification form 36d0655844 as per the instructions provided. This may involve mailing it to the relevant authority or submitting it online.
09
Keep a copy of the filled-out form and any supporting documents for your records.
10
Depending on the process, you may need to pay any applicable fees associated with the CLIA certification.
11
Wait for the certification authority to review your application. If approved, you will receive your CLIA certification.
Who needs clia certification 36d0655844?
01
Individuals or entities involved in clinical laboratories or diagnostic testing facilities generally need CLIA certification 36d0655844. This includes medical professionals, laboratory technicians, healthcare facilities, research institutions, and other relevant organizations. CLIA certification ensures compliance with quality standards and regulations set by the Clinical Laboratory Improvement Amendments (CLIA) program in the United States.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I get clia certification 36d0655844?
The premium version of pdfFiller gives you access to a huge library of fillable forms (more than 25 million fillable templates). You can download, fill out, print, and sign them all. State-specific clia certification 36d0655844 and other forms will be easy to find in the library. Find the template you need and use advanced editing tools to make it your own.
How do I make changes in clia certification 36d0655844?
pdfFiller not only lets you change the content of your files, but you can also change the number and order of pages. Upload your clia certification 36d0655844 to the editor and make any changes in a few clicks. The editor lets you black out, type, and erase text in PDFs. You can also add images, sticky notes, and text boxes, as well as many other things.
How can I edit clia certification 36d0655844 on a smartphone?
You can easily do so with pdfFiller's apps for iOS and Android devices, which can be found at the Apple Store and the Google Play Store, respectively. You can use them to fill out PDFs. We have a website where you can get the app, but you can also get it there. When you install the app, log in, and start editing clia certification 36d0655844, you can start right away.
What is clia certification 36d0655844?
CLIA certification 36d0655844 refers to a specific certification issued under the Clinical Laboratory Improvement Amendments (CLIA), which regulates laboratory testing and ensures the accuracy and reliability of test results.
Who is required to file clia certification 36d0655844?
All clinical laboratories that perform tests on human specimens for health assessment, diagnosis, prevention, or treatment purposes are required to file for CLIA certification 36d0655844.
How to fill out clia certification 36d0655844?
To fill out CLIA certification 36d0655844, you need to complete the application form with laboratory information, ownership details, types of tests performed, and any other required documentation as specified by the Centers for Medicare & Medicaid Services (CMS).
What is the purpose of clia certification 36d0655844?
The purpose of CLIA certification 36d0655844 is to ensure that clinical laboratories meet federal quality standards, thus ensuring the reliability of laboratory testing and protecting patient health.
What information must be reported on clia certification 36d0655844?
Information that must be reported includes laboratory name, address, director, types of tests performed, personnel qualifications, and compliance with quality control procedures.
Fill out your clia certification 36d0655844 online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Clia Certification 36D0655844 is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.




















