Get the free Biomedical Engineering Safety Concern form - bioe psu
Show details
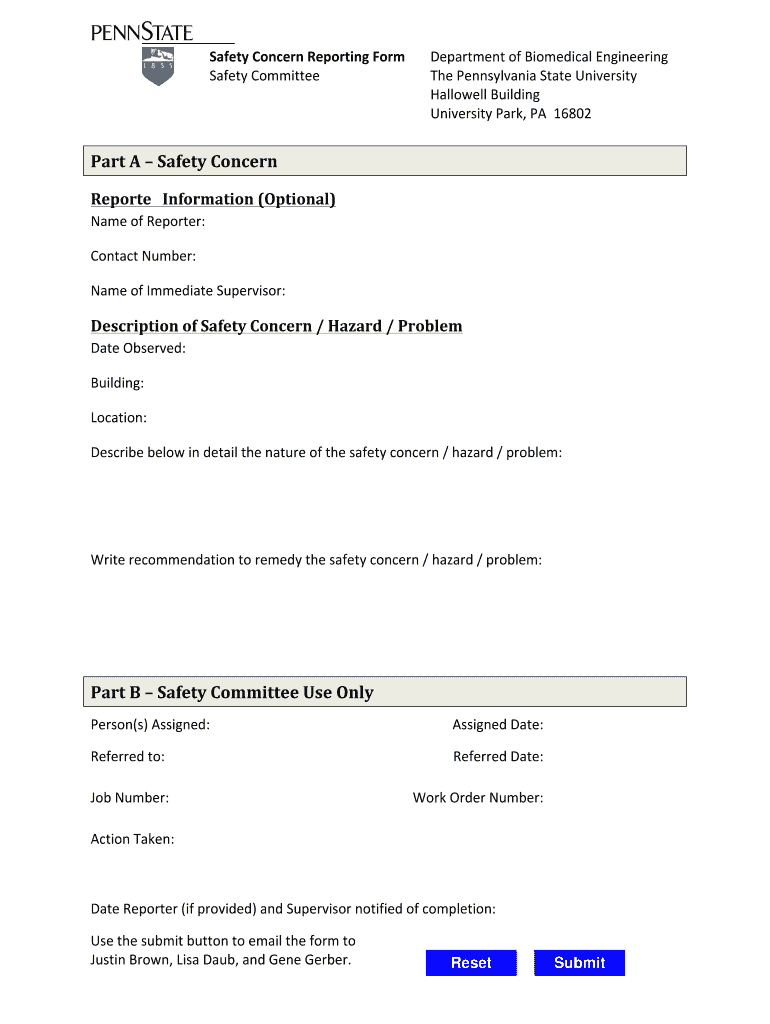
Safety Concern Reporting Form Safety Committee Department of Biomedical Engineering The Pennsylvania State University Hallow ell Building University Park, PA 16802 Part A Safety Concern Report Information
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign biomedical engineering safety concern

Edit your biomedical engineering safety concern form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your biomedical engineering safety concern form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing biomedical engineering safety concern online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Upload a file. Select Add New on your Dashboard and upload a file from your device or import it from the cloud, online, or internal mail. Then click Edit.
3
Edit biomedical engineering safety concern. Replace text, adding objects, rearranging pages, and more. Then select the Documents tab to combine, divide, lock or unlock the file.
4
Save your file. Choose it from the list of records. Then, shift the pointer to the right toolbar and select one of the several exporting methods: save it in multiple formats, download it as a PDF, email it, or save it to the cloud.
pdfFiller makes dealing with documents a breeze. Create an account to find out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out biomedical engineering safety concern

To fill out a biomedical engineering safety concern, follow these steps:
01
Start by identifying the specific safety concern that needs to be addressed. This could include issues related to equipment malfunction, hazardous materials, or potential risks to patient safety.
02
Gather all relevant information about the safety concern, such as the location where it occurred, the individuals involved, and any observations or documentation related to the incident.
03
Assess the severity of the safety concern. Determine the potential risks and the impact it may have on patients, healthcare professionals, or the overall functioning of the biomedical engineering department.
04
Consult with other relevant stakeholders, such as biomedical engineers, technicians, healthcare providers, and administrative personnel, to gather their insights and perspectives on the safety concern.
05
Document all the details of the safety concern in a clear and concise manner. Use a standardized reporting form or template, if available, to ensure consistency and completeness. Include dates, times, and specific descriptions of the incident.
06
Prioritize the safety concern based on its potential risks and urgency. Determine if immediate action is required or if it can be addressed as part of a larger safety improvement initiative.
07
Develop an action plan to address the safety concern. This may include implementing corrective measures, conducting further investigations, or updating policies and procedures to prevent similar incidents from occurring in the future.
08
Assign responsibility for each action item within the action plan. Clearly define who will be responsible for implementing each step and establish a timeline for completion.
09
Communicate the safety concern to all relevant parties, including supervisors, managers, and other healthcare professionals who may be impacted. Provide them with a copy of the documented safety concern and the action plan.
10
Continuously monitor and evaluate the progress of the action plan. Regularly communicate updates and ensure that all necessary steps are being taken to address the safety concern effectively.
Who needs to be concerned about biomedical engineering safety concerns?
Biomedical engineering safety concern should be a priority for the following individuals and entities:
01
Biomedical engineers and technicians: As professionals responsible for the design, maintenance, and repair of medical equipment, they must be vigilant in identifying and addressing any safety concerns that arise.
02
Healthcare providers: Doctors, nurses, and other healthcare professionals who use medical devices and equipment need to be aware of any safety concerns to ensure the well-being of their patients.
03
Hospital administrators and management: These individuals are responsible for overseeing the overall safety and quality of healthcare delivery. They need to be informed about any biomedical engineering safety concerns to make informed decisions and allocate resources appropriately.
04
Regulatory authorities: Government agencies responsible for overseeing biomedical engineering and healthcare quality, such as the Food and Drug Administration (FDA), may need to be notified of significant safety concerns that require their intervention or guidance.
05
Patients and their families: It is essential for patients and their families to be informed about any safety concerns that may impact their healthcare. This allows them to actively participate in their care and make informed decisions.
By involving all these stakeholders, a comprehensive approach towards biomedical engineering safety concerns can be achieved, leading to a safer and more reliable healthcare environment.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I send biomedical engineering safety concern to be eSigned by others?
When your biomedical engineering safety concern is finished, send it to recipients securely and gather eSignatures with pdfFiller. You may email, text, fax, mail, or notarize a PDF straight from your account. Create an account today to test it.
Can I edit biomedical engineering safety concern on an iOS device?
You certainly can. You can quickly edit, distribute, and sign biomedical engineering safety concern on your iOS device with the pdfFiller mobile app. Purchase it from the Apple Store and install it in seconds. The program is free, but in order to purchase a subscription or activate a free trial, you must first establish an account.
How do I fill out biomedical engineering safety concern on an Android device?
On Android, use the pdfFiller mobile app to finish your biomedical engineering safety concern. Adding, editing, deleting text, signing, annotating, and more are all available with the app. All you need is a smartphone and internet.
What is biomedical engineering safety concern?
Biomedical engineering safety concern involves identifying and addressing potential risks associated with medical devices, equipment, and procedures to ensure patient and user safety.
Who is required to file biomedical engineering safety concern?
Any individual or organization involved in the design, development, manufacturing, or use of biomedical equipment or devices may be required to file a safety concern.
How to fill out biomedical engineering safety concern?
To fill out a biomedical engineering safety concern, one must provide detailed information about the safety issue, including its description, potential impact, and proposed solutions.
What is the purpose of biomedical engineering safety concern?
The purpose of biomedical engineering safety concern is to promote the safe and effective use of medical devices and equipment to protect patients and users from harm.
What information must be reported on biomedical engineering safety concern?
Information that must be reported on a biomedical engineering safety concern includes the nature of the safety issue, potential hazards, affected products, and recommended corrective actions.
Fill out your biomedical engineering safety concern online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Biomedical Engineering Safety Concern is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















