Get the free 14th EudraVigilance Information Day - SwAPP - swapp
Show details
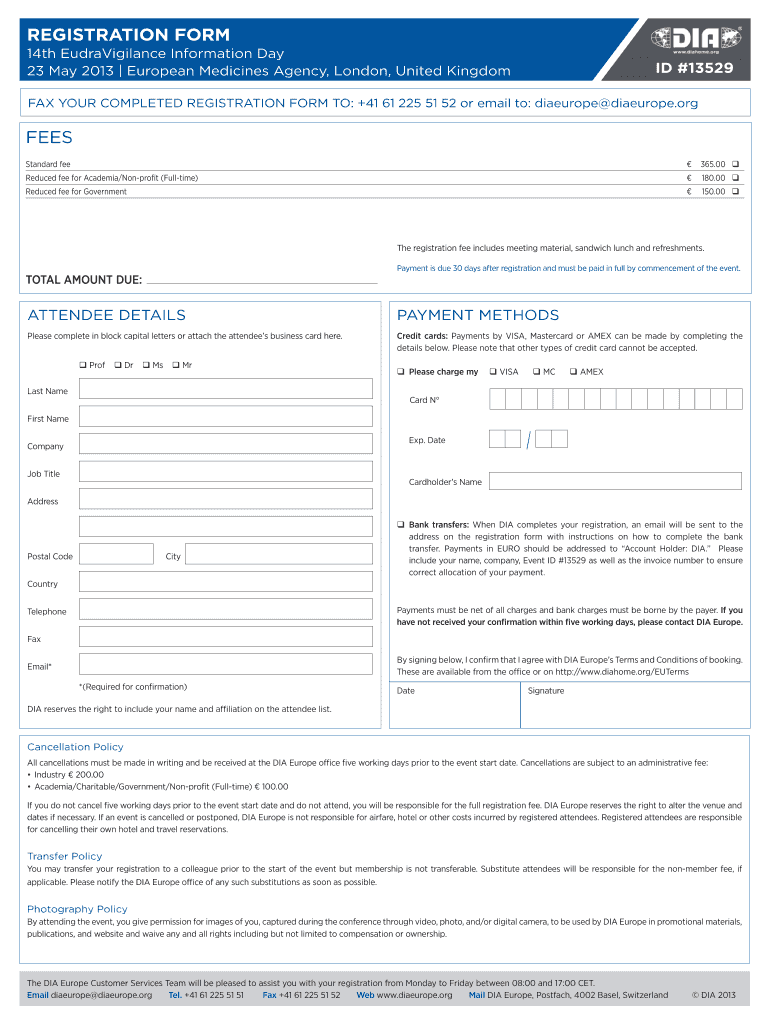
14th EudraVigilance Information Day Adverse drug reaction reporting in the EU and highlights of the new pharmacovigilance legislation Event #13529 23 May 2013 European Medicines Agency, London, United
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign 14th eudravigilance information day

Edit your 14th eudravigilance information day form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your 14th eudravigilance information day form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit 14th eudravigilance information day online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Log in to your account. Click Start Free Trial and sign up a profile if you don't have one yet.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit 14th eudravigilance information day. Rearrange and rotate pages, insert new and alter existing texts, add new objects, and take advantage of other helpful tools. Click Done to apply changes and return to your Dashboard. Go to the Documents tab to access merging, splitting, locking, or unlocking functions.
4
Save your file. Select it from your list of records. Then, move your cursor to the right toolbar and choose one of the exporting options. You can save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud, among other things.
Dealing with documents is simple using pdfFiller.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out 14th eudravigilance information day

How to fill out 14th Eudravigilance Information Day:
01
Start by familiarizing yourself with the purpose and objectives of the event. Understand the topics that will be covered and the intended audience.
02
Register for the event by visiting the official website or contacting the organizers. Provide all the required information accurately and make sure to meet any deadlines for registration.
03
Plan your schedule for the day, reviewing the agenda and selecting the sessions and presentations that are most relevant to your interests and needs.
04
Prepare any necessary documents or materials beforehand, such as a notebook, pen, and business cards.
05
On the day of the event, arrive early to allow yourself time to check in and find your way around. Follow any instructions given by the organizers and be respectful of other participants.
06
Take notes during the sessions that you attend, highlighting key points and any questions or ideas that arise. Engage in discussions and networking opportunities to make the most of the event.
07
After the event, reflect on what you have learned and consider how you can apply it to your work or research. Follow up with any contacts you made during the event to maintain and establish professional relationships.
Who needs 14th Eudravigilance Information Day:
01
Regulatory professionals working in the pharmaceutical industry who are responsible for pharmacovigilance and drug safety.
02
Healthcare professionals, such as doctors, pharmacists, and nurses, who are interested in staying up-to-date with the latest information on drug safety and adverse event reporting.
03
Researchers and academics in the field of pharmacology and drug development who want to enhance their knowledge and understanding of pharmacovigilance.
04
Representatives from regulatory authorities and organizations involved in drug regulation and monitoring.
05
Pharmaceutical company employees involved in drug safety and post-marketing surveillance.
06
Patient safety advocates and organizations interested in monitoring and improving the safety of medicinal products.
07
Anyone with a general interest in pharmacovigilance and drug safety, including students and the general public who want to learn more about this important aspect of healthcare.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
Where do I find 14th eudravigilance information day?
The premium pdfFiller subscription gives you access to over 25M fillable templates that you can download, fill out, print, and sign. The library has state-specific 14th eudravigilance information day and other forms. Find the template you need and change it using powerful tools.
How do I make edits in 14th eudravigilance information day without leaving Chrome?
Get and add pdfFiller Google Chrome Extension to your browser to edit, fill out and eSign your 14th eudravigilance information day, which you can open in the editor directly from a Google search page in just one click. Execute your fillable documents from any internet-connected device without leaving Chrome.
Can I edit 14th eudravigilance information day on an iOS device?
No, you can't. With the pdfFiller app for iOS, you can edit, share, and sign 14th eudravigilance information day right away. At the Apple Store, you can buy and install it in a matter of seconds. The app is free, but you will need to set up an account if you want to buy a subscription or start a free trial.
What is 14th eudravigilance information day?
The 14th EudraVigilance Information Day is an event organized to bring together stakeholders to discuss developments in pharmacovigilance and the electronic reporting of individual case safety reports (ICSRs) within the European Union.
Who is required to file 14th eudravigilance information day?
Pharmaceutical companies, regulatory authorities, healthcare professionals, and other stakeholders involved in pharmacovigilance are required to participate in the 14th EudraVigilance Information Day.
How to fill out 14th eudravigilance information day?
Participants can register for the event through the official website and attend the various sessions and workshops to learn about the latest updates and best practices in pharmacovigilance.
What is the purpose of 14th eudravigilance information day?
The purpose of the 14th EudraVigilance Information Day is to promote the safe and effective use of medicines by enhancing pharmacovigilance activities and fostering collaboration among stakeholders.
What information must be reported on 14th eudravigilance information day?
Participants are expected to share their experiences, challenges, and successes in pharmacovigilance, as well as discuss the importance of timely and accurate reporting of adverse drug reactions.
Fill out your 14th eudravigilance information day online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

14th Eudravigilance Information Day is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.


















