Get the free Clinical Trials Workshop II - swapp
Show details
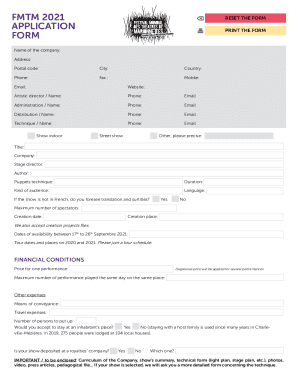
Clinical Trials Workshop II ? Translating the New Transparency Requirements into Practice Event #14116 24-25 September 2014 Millennium Gloucester Hotel London Kensington, UK Program Co-Chairs Sabine
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign clinical trials workshop ii

Edit your clinical trials workshop ii form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your clinical trials workshop ii form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing clinical trials workshop ii online
To use our professional PDF editor, follow these steps:
1
Create an account. Begin by choosing Start Free Trial and, if you are a new user, establish a profile.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit clinical trials workshop ii. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
With pdfFiller, it's always easy to work with documents. Try it out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out clinical trials workshop ii

How to fill out clinical trials workshop ii?
01
Start by carefully reading the instructions provided for the workshop. Understand the purpose and objective of the workshop to get a clear idea of what is expected.
02
Take note of any prerequisites or requirements mentioned. Ensure that you meet all the necessary criteria to participate in the workshop.
03
Gather all the required documents and information needed for the workshop. This may include personal identification, medical history, and any relevant research or clinical trial experience.
04
Fill out the registration form accurately and completely. Ensure that all the information provided is correct and up to date. Double-check for any errors or missing details.
05
Pay attention to any specific instructions regarding payment for the workshop. Follow the designated payment method and deadline set by the organizers.
Who needs clinical trials workshop ii?
01
Individuals interested in the field of clinical research and trials can benefit from attending the clinical trials workshop ii. This includes researchers, scientists, medical professionals, and students pursuing a career in health sciences.
02
Professionals already involved in clinical trials who want to enhance their knowledge and skills can find value in attending the workshop. The workshop may provide updates on industry standards, best practices, and new advancements in clinical trials.
03
Individuals involved in the regulatory or ethics aspects of clinical trials may also benefit from attending the workshop. This can include professionals working in regulatory agencies, ethics committees, or organizations overseeing the conduct of clinical trials.
In summary, anyone with an interest in clinical trials, whether they are beginners or experienced professionals, can benefit from participating in the clinical trials workshop ii. The workshop provides valuable insights, knowledge, and updates in the field of clinical research.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I edit clinical trials workshop ii from Google Drive?
Using pdfFiller with Google Docs allows you to create, amend, and sign documents straight from your Google Drive. The add-on turns your clinical trials workshop ii into a dynamic fillable form that you can manage and eSign from anywhere.
How do I make changes in clinical trials workshop ii?
pdfFiller allows you to edit not only the content of your files, but also the quantity and sequence of the pages. Upload your clinical trials workshop ii to the editor and make adjustments in a matter of seconds. Text in PDFs may be blacked out, typed in, and erased using the editor. You may also include photos, sticky notes, and text boxes, among other things.
How do I fill out the clinical trials workshop ii form on my smartphone?
You can quickly make and fill out legal forms with the help of the pdfFiller app on your phone. Complete and sign clinical trials workshop ii and other documents on your mobile device using the application. If you want to learn more about how the PDF editor works, go to pdfFiller.com.
What is clinical trials workshop ii?
Clinical trials workshop ii is a training event designed to educate and provide guidance on the conduct of clinical trials.
Who is required to file clinical trials workshop ii?
Researchers, clinical trial sponsors, and individuals involved in the management of clinical trials are required to file clinical trials workshop ii.
How to fill out clinical trials workshop ii?
Clinical trials workshop ii can be filled out online or in paper form, and must include accurate information about the trial protocol, participants, outcomes, and any adverse events.
What is the purpose of clinical trials workshop ii?
The purpose of clinical trials workshop ii is to ensure that clinical trials are conducted in a safe and ethical manner, following regulatory guidelines.
What information must be reported on clinical trials workshop ii?
Information such as trial protocol, participant demographics, outcomes, adverse events, and protocol deviations must be reported on clinical trials workshop ii.
Fill out your clinical trials workshop ii online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Clinical Trials Workshop Ii is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.