Get the free olympus scope cleaning competency
Fill out, sign, and share forms from a single PDF platform
Edit and sign in one place
Create professional forms
Simplify data collection
Manage forms centrally
Why pdfFiller is the best tool for your documents and forms
End-to-end document management
Accessible from anywhere
Secure and compliant
Understanding the Olympus Scope Cleaning Competency Form
Overview of the Olympus Scope Cleaning Competency Form
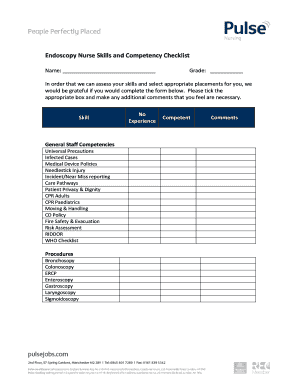
The Olympus scope cleaning competency form is a critical document utilized to ensure that personnel are trained in the proper cleaning and maintenance protocols for Olympus scopes. This form serves to verify the competencies of staff engaged in the cleaning process, promoting safety and quality in medical settings. The essential goal is to maintain high standards in endoscope cleanliness, thereby protecting patient health.
Key Features of the Olympus Scope Cleaning Competency Form
The form includes various key features, such as sections for user identification, a checklist for cleaning procedures, and areas for notes and feedback. It provides detailed instructions aligned with Olympus's instructional materials, ensuring comprehensive coverage of all necessary steps. This promotes accountability by tracking competencies over time.
Best Practices for Accurate Completion
Completing the Olympus scope cleaning competency form accurately is vital for compliance and patient safety. Best practices include reviewing the form thoroughly before filling it out, following the procedural steps as outlined, and confirming all entries for clarity and correctness. Peer review can be beneficial to ensure all aspects have been captured properly.
Who Needs the Olympus Scope Cleaning Competency Form
This form is essential for all healthcare professionals involved in the cleaning and maintenance of Olympus scopes. This includes nurses, technicians, and any other personnel responsible for endoscope reprocessing. Additionally, supervisors and quality assurance staff may utilize the form to evaluate overall compliance with cleaning protocols.
How to Fill the Olympus Scope Cleaning Competency Form
Filling out the form requires careful attention to detail. Begin by entering personal and professional information in the designated fields. Follow the checklist items sequentially, documenting each step taken during the cleaning process. Record observations, challenges, or notes on the form for continuous improvement. Ensure to sign and date the form upon completion.
Security and Compliance for the Olympus Scope Cleaning Competency Form
Security and compliance are paramount in handling the Olympus scope cleaning competency form. All entries must be treated as confidential and stored securely. Staff should adhere to relevant regulations and policies regarding documentation and patient safety to ensure compliance with both internal and external standards.
Frequently Asked Questions about checklist olympus endoscope cleaning poster form
What types of medical professionals should use the Olympus scope cleaning competency form?
The Olympus scope cleaning competency form should be used by any medical professionals involved in the cleaning and maintenance of Olympus scopes, such as nurses and technicians.
Why is accurate completion of the form critical?
Accurate completion of the form is essential to ensure safety, compliance with cleaning protocols, and tracking of personnel competencies in the maintenance of medical equipment.
pdfFiller scores top ratings on review platforms