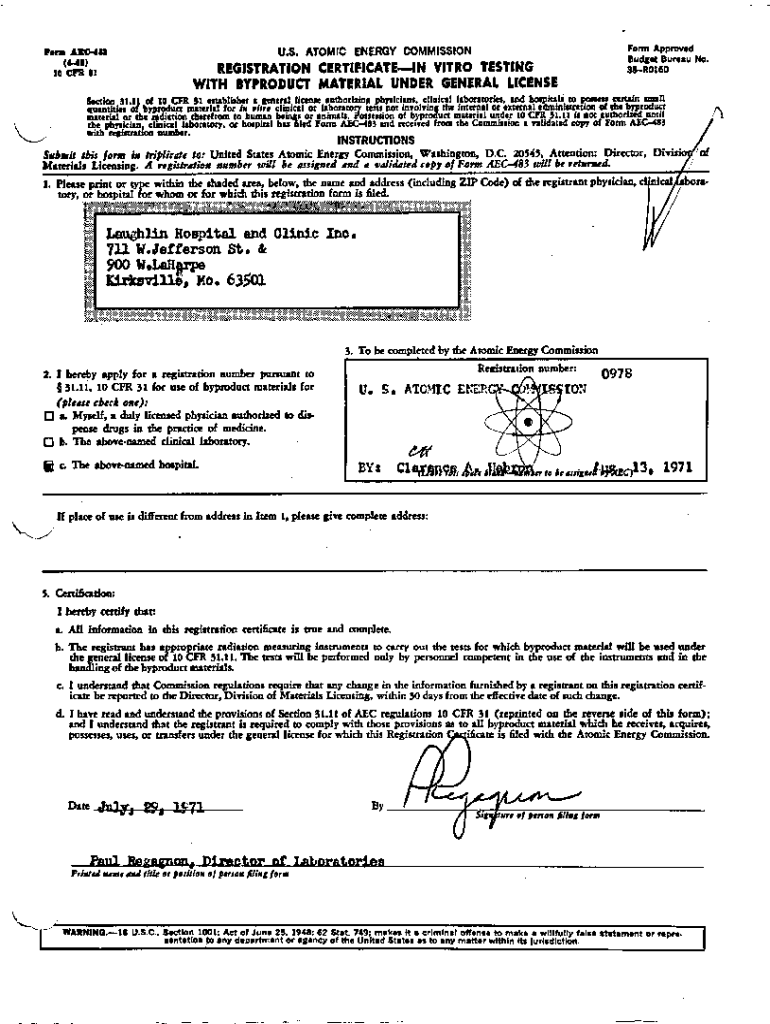
Get the free Registration Certificate for In-Vitro Testing for Laughlin Hospital and Clinic, Inc.
Show details
Form A3C481(448)inform AP;)node. S. ATOMIC ENERGY COMMISSIONelursou No. VT Budget
REGISTRATION CERTIFICATE IN VITO TESTING
WITH BYPRODUCT MATERIAL UNDER GENERAL LICENSE
nfsiimAaasERf55p35R0160Section
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign registration certificate for in-vitro

Edit your registration certificate for in-vitro form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your registration certificate for in-vitro form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing registration certificate for in-vitro online
Use the instructions below to start using our professional PDF editor:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit registration certificate for in-vitro. Text may be added and replaced, new objects can be included, pages can be rearranged, watermarks and page numbers can be added, and so on. When you're done editing, click Done and then go to the Documents tab to combine, divide, lock, or unlock the file.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
pdfFiller makes working with documents easier than you could ever imagine. Try it for yourself by creating an account!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out registration certificate for in-vitro

How to fill out registration certificate for in-vitro
01
To fill out a registration certificate for in-vitro, follow these steps:
02
Start by writing the name and address of the facility or organization that is applying for the certificate.
03
Provide details about the type of in-vitro diagnostic product that needs to be registered.
04
Include information about the manufacturer or supplier of the product.
05
Specify the intended use of the product and its classification according to the relevant regulations.
06
Provide a list of technical documentation and supporting evidence to demonstrate the safety and effectiveness of the product.
07
Include information about any clinical trials or studies conducted for the product, if applicable.
08
Clearly state any proposed labeling or instructions for use that will be provided with the product.
09
Provide contact information for the person responsible for the application, including name, address, phone number, and email.
10
Sign and date the registration certificate application.
11
Submit the completed application along with any required fees or supporting documents to the appropriate regulatory authority.
12
Keep a copy of the application and any submission receipts for your records.
13
Wait for a response from the regulatory authority regarding the status of your application.
Who needs registration certificate for in-vitro?
01
The following entities may need a registration certificate for in-vitro:
02
- Manufacturers or suppliers of in-vitro diagnostic products.
03
- Facilities or organizations that conduct clinical trials or studies involving in-vitro diagnostic products.
04
- Healthcare institutions or laboratories that use in-vitro diagnostic products for patient testing or diagnosis.
05
- Regulatory authorities or agencies responsible for overseeing the safety and quality of in-vitro diagnostic products.
06
It is important to consult the relevant regulations and guidelines in your country or region to determine if a registration certificate for in-vitro is required for your specific situation.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I get registration certificate for in-vitro?
It’s easy with pdfFiller, a comprehensive online solution for professional document management. Access our extensive library of online forms (over 25M fillable forms are available) and locate the registration certificate for in-vitro in a matter of seconds. Open it right away and start customizing it using advanced editing features.
Can I create an electronic signature for the registration certificate for in-vitro in Chrome?
As a PDF editor and form builder, pdfFiller has a lot of features. It also has a powerful e-signature tool that you can add to your Chrome browser. With our extension, you can type, draw, or take a picture of your signature with your webcam to make your legally-binding eSignature. Choose how you want to sign your registration certificate for in-vitro and you'll be done in minutes.
Can I create an eSignature for the registration certificate for in-vitro in Gmail?
With pdfFiller's add-on, you may upload, type, or draw a signature in Gmail. You can eSign your registration certificate for in-vitro and other papers directly in your mailbox with pdfFiller. To preserve signed papers and your personal signatures, create an account.
What is registration certificate for in-vitro?
A registration certificate for in-vitro is an official document that certifies that a facility or entity is authorized to conduct in-vitro diagnostic tests or procedures.
Who is required to file registration certificate for in-vitro?
Entities that manufacture, import, or distribute in-vitro diagnostic devices or perform in-vitro testing are required to file a registration certificate.
How to fill out registration certificate for in-vitro?
To fill out the registration certificate for in-vitro, you must provide specific details about your facility, the types of in-vitro diagnostic devices you handle, and comply with the regulatory requirements set by the relevant authorities.
What is the purpose of registration certificate for in-vitro?
The purpose of the registration certificate for in-vitro is to ensure that entities comply with safety and regulatory standards, maintain quality in diagnostics, and protect public health.
What information must be reported on registration certificate for in-vitro?
Information required on the registration certificate typically includes facility name, address, type of in-vitro diagnostics performed, and compliance with regulatory standards.
Fill out your registration certificate for in-vitro online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Registration Certificate For In-Vitro is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.




















