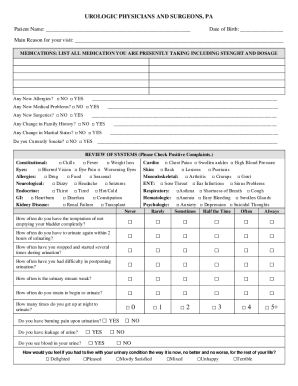
Get the free Principal Investigator’s Handbook - shu
Show details
El Handbook del Investigador Principal está diseñado para introducir a los miembros de la facultad y administradores al proceso de obtención de financiamiento y los procedimientos que rigen las
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign principal investigators handbook
Edit your principal investigators handbook form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.
Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your principal investigators handbook form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing principal investigators handbook online
To use our professional PDF editor, follow these steps:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Upload a file. Select Add New on your Dashboard and upload a file from your device or import it from the cloud, online, or internal mail. Then click Edit.
3
Edit principal investigators handbook. Replace text, adding objects, rearranging pages, and more. Then select the Documents tab to combine, divide, lock or unlock the file.
4
Get your file. Select the name of your file in the docs list and choose your preferred exporting method. You can download it as a PDF, save it in another format, send it by email, or transfer it to the cloud.
pdfFiller makes working with documents easier than you could ever imagine. Try it for yourself by creating an account!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out principal investigators handbook
How to fill out Principal Investigator’s Handbook
01
Begin by reviewing the table of contents to understand the structure of the handbook.
02
Read the introduction to grasp the purpose and importance of the handbook.
03
Follow the guidelines in each section, starting with the eligibility criteria for principal investigators.
04
Fill out the required forms and compile necessary documentation as indicated.
05
Ensure compliance with institutional policies and funding agency requirements.
06
Review submission timelines and adhere to deadlines provided in the handbook.
07
Seek assistance from your institution's research office if you have questions or need clarification.
08
Make sure to proofread your entries before finalizing the documents.
Who needs Principal Investigator’s Handbook?
01
Researchers who are applying for grants or conducting research projects.
02
University faculty members serving as principal investigators.
03
Administrative staff involved in research management and compliance.
04
Students pursuing research under the supervision of a principal investigator.
05
Institutional review boards and funding agencies who need to assess research proposals.
Fill
form
: Try Risk Free






People Also Ask about
How much does a principal investigator make at NIH?
Educational Background: Principal Investigators (PIs) in clinical trials must hold a medical or doctoral degree in a relevant field, ensuring a strong foundation in the scientific and medical aspects of the research.
What is a principal investigator in English?
The person(s) in charge of a clinical trial or a scientific research grant. The principal investigator prepares and carries out the clinical trial protocol (plan for the study) or research paid for by the grant. The principal investigator also analyzes the data and reports the results of the trial or grant research.
What is the principal investigator toolkit?
The tool kit works to create a patient-driven message, with a clinical trial patient advocate working with the principal investigator to cultivate a message that resonates with the patient community.
Can a non-physician be a principal investigator?
A PI need not be a physician. Sponsors who design and pay for clinical trials (eg, pharmaceutical companies) are required by law6,7 to select individuals who are qualified by training and experience to conduct a clinical trial.
What does the principal investigator do?
Principal Investigator (PI) – A Principal Investigator is the primary individual responsible for the preparation, conduct, and administration of a research grant, cooperative agreement, training or public service project, contract, or other sponsored project in compliance with applicable laws and regulations and
What is principal investigator GLP?
The Principal Investigator will ensure that the delegated phases of the study are conducted in ance with the applicable Principles of Good Laboratory Practice.
Do you need a PhD to be a principal investigator?
Educational Background: Principal Investigators (PIs) in clinical trials must hold a medical or doctoral degree in a relevant field, ensuring a strong foundation in the scientific and medical aspects of the research.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is Principal Investigator’s Handbook?
The Principal Investigator’s Handbook is a comprehensive guide designed to provide essential information and best practices for principal investigators managing research projects, ensuring compliance with regulations and institutional policies.
Who is required to file Principal Investigator’s Handbook?
Principal Investigators (PIs) involved in conducting research projects that require institutional oversight and funding are typically required to file the Principal Investigator’s Handbook.
How to fill out Principal Investigator’s Handbook?
To fill out the Principal Investigator’s Handbook, PIs should follow the outlined sections, providing accurate details on their research proposal, funding sources, conflict of interest disclosures, and safety protocols, while adhering to institutional guidelines.
What is the purpose of Principal Investigator’s Handbook?
The purpose of the Principal Investigator’s Handbook is to ensure that PIs understand their responsibilities, comply with ethical and regulatory standards, and effectively manage their research projects to promote integrity and accountability.
What information must be reported on Principal Investigator’s Handbook?
The Principal Investigator’s Handbook must report information such as the project's title, funding details, research objectives, PI's qualifications, team members, compliance with ethical standards, and safety measures.
Fill out your principal investigators handbook online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Principal Investigators Handbook is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.