Get the free Randomized Controlled Trial of Three Different Learning Formats to ...
Show details
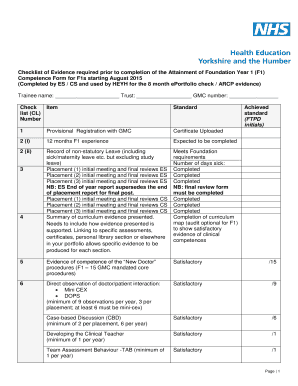
AD AWARD NUMBER: DM150022TITLE: RANDOMIZEDCONTROLLED TRIAL OF THREE DIFFERENT LEARNING FORMATS TO TEACH THE UNIVERSAL PROTOCOL AND TIMEOUTSPRINCIPAL INVESTIGATOR:Douglas E. Paul, MD, RECIPIENT: VA
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign randomized controlled trial of

Edit your randomized controlled trial of form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your randomized controlled trial of form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit randomized controlled trial of online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Create an account. Begin by choosing Start Free Trial and, if you are a new user, establish a profile.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit randomized controlled trial of. Add and change text, add new objects, move pages, add watermarks and page numbers, and more. Then click Done when you're done editing and go to the Documents tab to merge or split the file. If you want to lock or unlock the file, click the lock or unlock button.
4
Save your file. Select it from your list of records. Then, move your cursor to the right toolbar and choose one of the exporting options. You can save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud, among other things.
pdfFiller makes dealing with documents a breeze. Create an account to find out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out randomized controlled trial of

How to fill out randomized controlled trial of
01
To fill out a randomized controlled trial, follow these steps:
02
Identify the research question or hypothesis that you want to investigate.
03
Design the trial, including determining the study population, intervention or treatment, control group, randomization method, and outcome measures.
04
Obtain ethical approval and any necessary permissions or licenses.
05
Recruit participants for the trial.
06
Randomize participants into either the intervention group or the control group.
07
Administer the intervention or treatment to the intervention group, while the control group receives either a placebo or standard care.
08
Collect data on the outcome measures from both groups.
09
Analyze the data using appropriate statistical methods.
10
Interpret the results and draw conclusions.
11
Report the findings in a scientific paper or presentation.
Who needs randomized controlled trial of?
01
Randomized controlled trials are needed by researchers, scientists, and medical professionals who want to evaluate the effectiveness and safety of a new intervention or treatment.
02
They are often used in the fields of medicine, public health, psychology, and social sciences to generate reliable evidence for making informed decisions and providing the best possible care or services to individuals or populations.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I make edits in randomized controlled trial of without leaving Chrome?
Install the pdfFiller Chrome Extension to modify, fill out, and eSign your randomized controlled trial of, which you can access right from a Google search page. Fillable documents without leaving Chrome on any internet-connected device.
How do I edit randomized controlled trial of straight from my smartphone?
Using pdfFiller's mobile-native applications for iOS and Android is the simplest method to edit documents on a mobile device. You may get them from the Apple App Store and Google Play, respectively. More information on the apps may be found here. Install the program and log in to begin editing randomized controlled trial of.
How do I complete randomized controlled trial of on an iOS device?
Download and install the pdfFiller iOS app. Then, launch the app and log in or create an account to have access to all of the editing tools of the solution. Upload your randomized controlled trial of from your device or cloud storage to open it, or input the document URL. After filling out all of the essential areas in the document and eSigning it (if necessary), you may save it or share it with others.
What is randomized controlled trial of?
A randomized controlled trial (RCT) is a scientific study that aims to evaluate the effectiveness of an intervention or treatment by randomly assigning participants into different groups - typically a treatment group and a control group - to measure the outcome.
Who is required to file randomized controlled trial of?
Researchers and organizations conducting the trial, including academic institutions, medical centers, and private companies, are typically required to register and report their randomized controlled trials.
How to fill out randomized controlled trial of?
To fill out a randomized controlled trial registration, researchers must provide detailed information including the trial's title, objectives, methodology, outcomes, and sponsor information, usually through a designated clinical trial registry.
What is the purpose of randomized controlled trial of?
The purpose of a randomized controlled trial is to eliminate bias in the results and to determine the causal effects of interventions by comparing the outcomes between the treatment and control groups.
What information must be reported on randomized controlled trial of?
Key information that must be reported includes the trial's title, start and end dates, eligibility criteria, detailed description of interventions, primary and secondary outcomes, and statistical methods used for analysis.
Fill out your randomized controlled trial of online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Randomized Controlled Trial Of is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.