Get the free Smlouva o klinickem hodnoceni FHoffmann - La Roche GA29103 FNHKredacted.doc - smlouv...
Show details
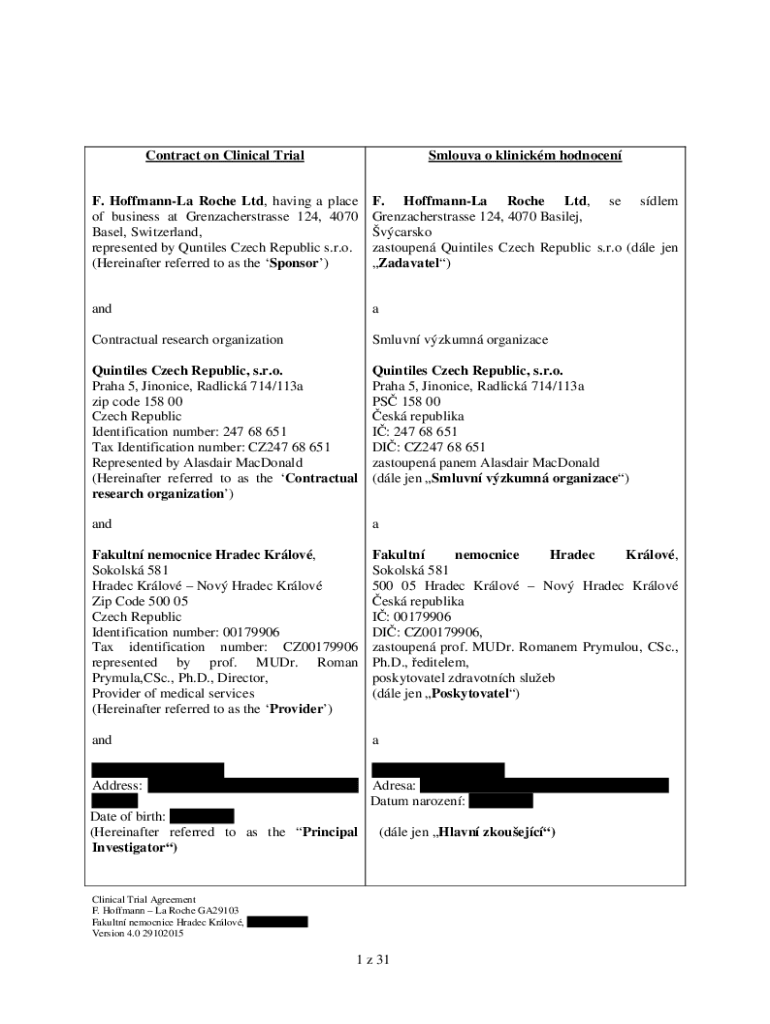
Contract on Clinical TrialSmlouva o clinical Holocene. Hoffmann Roche Ltd, having a place
of business at Grenzacherstrasse 124, 4070
Basel, Switzerland,
represented by Quantiles Czech Republic s.r.o.
(Hereinafter
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign smlouva o klinickem hodnoceni

Edit your smlouva o klinickem hodnoceni form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your smlouva o klinickem hodnoceni form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit smlouva o klinickem hodnoceni online
Use the instructions below to start using our professional PDF editor:
1
Create an account. Begin by choosing Start Free Trial and, if you are a new user, establish a profile.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit smlouva o klinickem hodnoceni. Rearrange and rotate pages, add new and changed texts, add new objects, and use other useful tools. When you're done, click Done. You can use the Documents tab to merge, split, lock, or unlock your files.
4
Get your file. When you find your file in the docs list, click on its name and choose how you want to save it. To get the PDF, you can save it, send an email with it, or move it to the cloud.
It's easier to work with documents with pdfFiller than you can have ever thought. You may try it out for yourself by signing up for an account.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out smlouva o klinickem hodnoceni

How to fill out smlouva o klinickem hodnoceni
01
Start by reading the entire smlouva o klinickem hodnoceni to understand its purpose and requirements.
02
Gather all the necessary information and documentation needed to complete the agreement, such as the names of the parties involved, details of the clinical evaluation, and any specific terms and conditions.
03
Begin by filling out the header of the agreement, which typically includes the date, the names and contact information of the parties involved, and the title of the agreement.
04
Proceed to the main body of the agreement, where you will need to provide a detailed description of the clinical evaluation being conducted, including the objectives, methodology, and timeline.
05
Include any additional clauses or provisions that may be required or relevant to the specific clinical evaluation being conducted.
06
Make sure to review the completed smlouva o klinickem hodnoceni for accuracy and clarity, ensuring that all the necessary information and terms are included and correctly stated.
07
Once you are satisfied with the content of the agreement, sign and date it, and have all the parties involved sign and date their respective sections.
08
Make copies of the signed agreement for all parties and keep the original document in a secure and easily accessible location.
09
It is always advisable to seek legal advice or consult with an expert in contract law when filling out smlouva o klinickem hodnoceni to ensure its compliance with applicable laws and regulations.
Who needs smlouva o klinickem hodnoceni?
01
Smlouva o klinickem hodnoceni is needed by individuals or organizations involved in conducting clinical evaluations or studies.
02
This may include pharmaceutical companies, researchers, medical institutions, and regulatory authorities.
03
It is a crucial legal document that outlines the terms, obligations, and responsibilities of all parties involved in the clinical evaluation process.
04
Having a properly executed smlouva o klinickem hodnoceni helps ensure transparency, compliance with regulations, and protection of the rights and interests of all involved parties.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I manage my smlouva o klinickem hodnoceni directly from Gmail?
smlouva o klinickem hodnoceni and other documents can be changed, filled out, and signed right in your Gmail inbox. You can use pdfFiller's add-on to do this, as well as other things. When you go to Google Workspace, you can find pdfFiller for Gmail. You should use the time you spend dealing with your documents and eSignatures for more important things, like going to the gym or going to the dentist.
How can I modify smlouva o klinickem hodnoceni without leaving Google Drive?
By combining pdfFiller with Google Docs, you can generate fillable forms directly in Google Drive. No need to leave Google Drive to make edits or sign documents, including smlouva o klinickem hodnoceni. Use pdfFiller's features in Google Drive to handle documents on any internet-connected device.
Can I create an electronic signature for the smlouva o klinickem hodnoceni in Chrome?
Yes. You can use pdfFiller to sign documents and use all of the features of the PDF editor in one place if you add this solution to Chrome. In order to use the extension, you can draw or write an electronic signature. You can also upload a picture of your handwritten signature. There is no need to worry about how long it takes to sign your smlouva o klinickem hodnoceni.
What is smlouva o klinickem hodnoceni?
Smlouva o klinickem hodnoceni, or clinical evaluation agreement, is a contract that outlines the terms and conditions for conducting clinical trials, detailing roles and responsibilities of parties involved.
Who is required to file smlouva o klinickem hodnoceni?
Sponsors of clinical trials, including pharmaceutical companies, medical device manufacturers, and researchers, are typically required to file a smlouva o klinickem hodnoceni.
How to fill out smlouva o klinickem hodnoceni?
To fill out a smlouva o klinickem hodnoceni, stakeholders must provide information about the trial objectives, responsibilities, timelines, financial arrangements, and compliance with regulatory requirements.
What is the purpose of smlouva o klinickem hodnoceni?
The purpose of smlouva o klinickem hodnoceni is to ensure transparency, protect the rights of participants, and establish the legal framework for conducting clinical research.
What information must be reported on smlouva o klinickem hodnoceni?
Key information that must be reported includes details of the clinical trial, including objectives, methodology, investigator information, participant safety measures, and compliance with ethical standards.
Fill out your smlouva o klinickem hodnoceni online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Smlouva O Klinickem Hodnoceni is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















