Get the free A Trial Investigating the Safety and Effects of One BNT162 ... - smlouvy gov
Show details
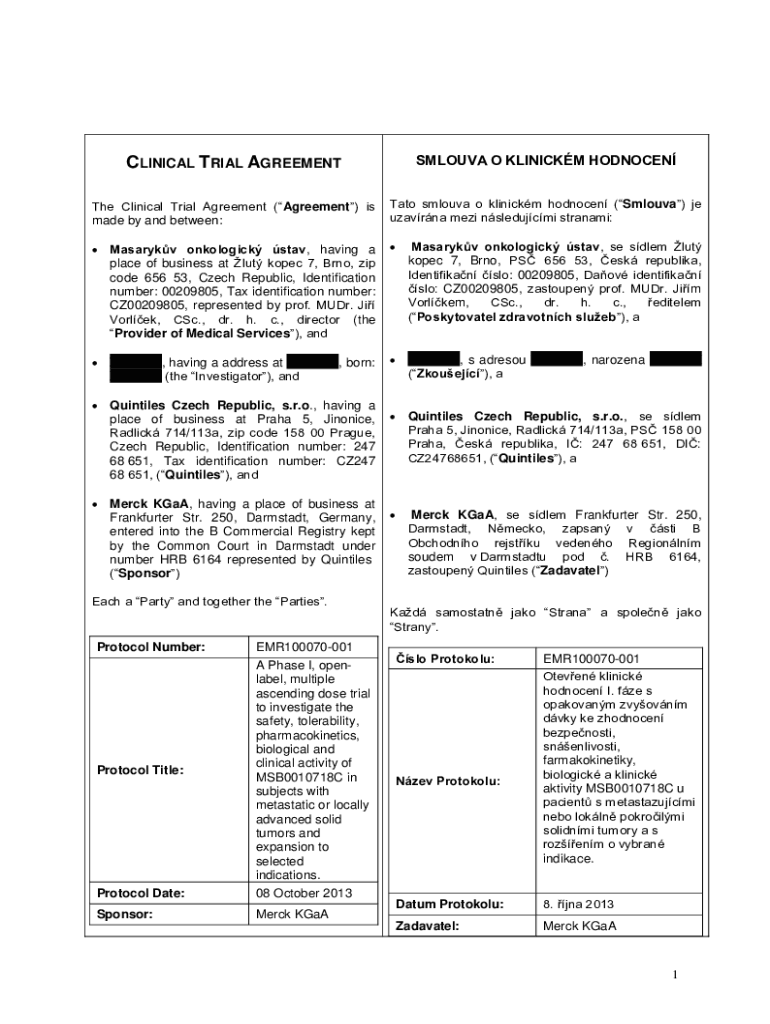
CLINICAL TRIAL AGREEMENTSMLOUVA O CLINICAL HODNOCENThe Clinical Trial Agreement (Agreement) is
made by and between:NATO Silva o clinical honored (Silva) JE
Havana meze nsledujcmi stream: Masaryk oncologic
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign a trial investigating form

Edit your a trial investigating form form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your a trial investigating form form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing a trial investigating form online
Use the instructions below to start using our professional PDF editor:
1
Log in. Click Start Free Trial and create a profile if necessary.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit a trial investigating form. Rearrange and rotate pages, add new and changed texts, add new objects, and use other useful tools. When you're done, click Done. You can use the Documents tab to merge, split, lock, or unlock your files.
4
Save your file. Select it from your list of records. Then, move your cursor to the right toolbar and choose one of the exporting options. You can save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud, among other things.
With pdfFiller, dealing with documents is always straightforward.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out a trial investigating form

How to fill out a trial investigating form
01
Start by reading the instructions on the trial investigating form.
02
Gather all the necessary information and documents needed to fill out the form.
03
Begin by filling out the basic information section, such as your name, contact details, and any relevant identification numbers.
04
Move on to the details of the trial investigation, providing information about the incident or situation being investigated.
05
Be precise and thorough when describing the events or circumstances, providing dates, times, locations, and any other pertinent details.
06
Include any supporting evidence or documentation, such as photographs, videos, or witness statements, if applicable.
07
Answer any specific questions or prompts provided on the form, ensuring that your responses are clear and concise.
08
Review the completed form for accuracy and make any necessary corrections or additions.
09
Sign and date the form, acknowledging that the information provided is true and accurate to the best of your knowledge.
10
Submit the completed trial investigating form to the designated recipient or authority, following any specified submission instructions.
Who needs a trial investigating form?
01
A trial investigating form may be required by various individuals or entities involved in a legal or disciplinary process, such as:
02
- Attorneys or legal representatives
03
- Law enforcement agencies
04
- Regulatory bodies or government agencies
05
- Human resources departments
06
- Compliance officers
07
- Individuals involved in the incident being investigated
08
- Witnesses or individuals with relevant information
09
The specific need for a trial investigating form will depend on the nature of the investigation and the requirements of the legal or disciplinary procedure.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I manage my a trial investigating form directly from Gmail?
It's easy to use pdfFiller's Gmail add-on to make and edit your a trial investigating form and any other documents you get right in your email. You can also eSign them. Take a look at the Google Workspace Marketplace and get pdfFiller for Gmail. Get rid of the time-consuming steps and easily manage your documents and eSignatures with the help of an app.
Where do I find a trial investigating form?
With pdfFiller, an all-in-one online tool for professional document management, it's easy to fill out documents. Over 25 million fillable forms are available on our website, and you can find the a trial investigating form in a matter of seconds. Open it right away and start making it your own with help from advanced editing tools.
How do I complete a trial investigating form online?
Completing and signing a trial investigating form online is easy with pdfFiller. It enables you to edit original PDF content, highlight, blackout, erase and type text anywhere on a page, legally eSign your form, and much more. Create your free account and manage professional documents on the web.
What is a trial investigating form?
A trial investigating form is a document used to collect and report data regarding a trial, including its design, methodology, and results.
Who is required to file a trial investigating form?
Researchers, sponsors, or institutions conducting clinical trials are generally required to file a trial investigating form.
How to fill out a trial investigating form?
To fill out a trial investigating form, you need to provide information about the trial's objectives, methodology, participant details, outcomes, and any adverse events, following the guidelines provided by regulatory authorities.
What is the purpose of a trial investigating form?
The purpose of a trial investigating form is to ensure compliance with regulatory standards, facilitate data collection, and provide comprehensive information about the trial to relevant stakeholders.
What information must be reported on a trial investigating form?
Information that must be reported includes trial title, objectives, design, participant demographics, intervention details, outcomes, and any incidence of adverse events.
Fill out your a trial investigating form online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

A Trial Investigating Form is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















