Get the free Study Evaluating the Efficacy, Safety, and Tolerability of ... - smlouvy gov
Show details
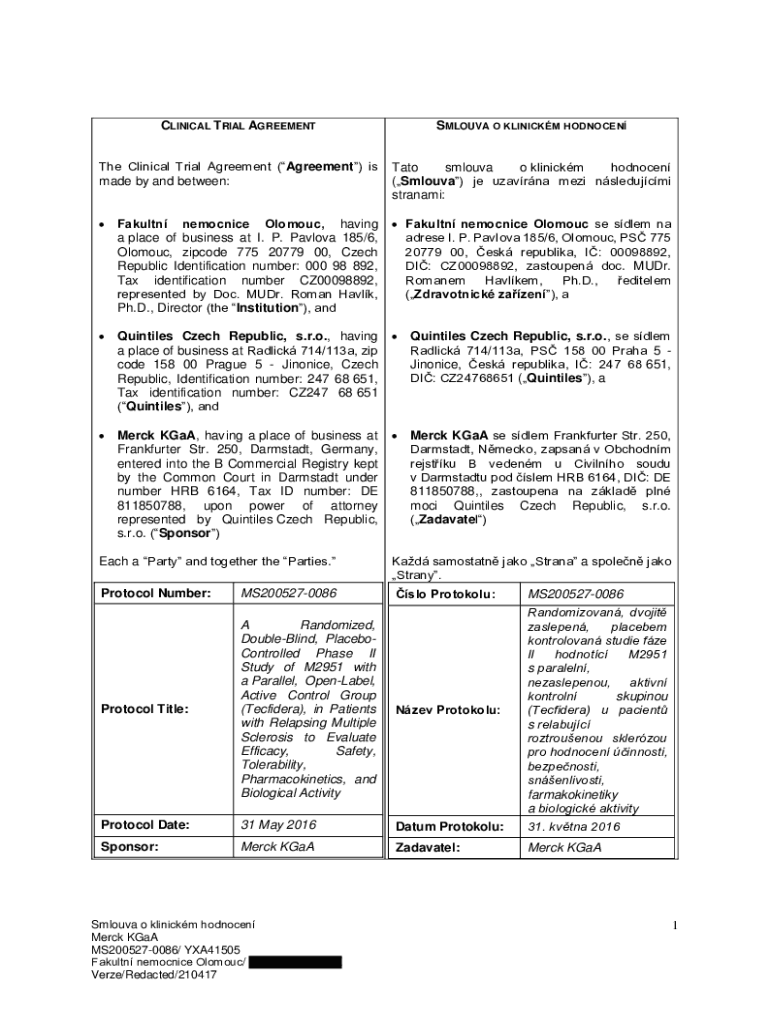
CLINICAL T RIAL AGREEMENTSMLOUVA O CLINICAL HODNOCENThe Clinical Trial Agreement (Agreement) is
made by and between:NATO
Silva
o clinical
honored
(Silva) JE Havana meze nsledujcmi
stream: Faculty
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign study evaluating form efficacy

Edit your study evaluating form efficacy form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your study evaluating form efficacy form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit study evaluating form efficacy online
Follow the steps down below to use a professional PDF editor:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Upload a file. Select Add New on your Dashboard and upload a file from your device or import it from the cloud, online, or internal mail. Then click Edit.
3
Edit study evaluating form efficacy. Rearrange and rotate pages, insert new and alter existing texts, add new objects, and take advantage of other helpful tools. Click Done to apply changes and return to your Dashboard. Go to the Documents tab to access merging, splitting, locking, or unlocking functions.
4
Get your file. When you find your file in the docs list, click on its name and choose how you want to save it. To get the PDF, you can save it, send an email with it, or move it to the cloud.
pdfFiller makes working with documents easier than you could ever imagine. Create an account to find out for yourself how it works!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out study evaluating form efficacy

How to fill out study evaluating form efficacy
01
Start by understanding the purpose of the study evaluating form efficacy.
02
Identify the key components and parameters that need to be evaluated in the form.
03
Develop a clear and concise set of instructions for filling out the form.
04
Provide examples or guidelines to ensure consistent and accurate responses.
05
Use a structured format with appropriate sections and headings for easy navigation.
06
Include a rating or scoring system to quantify the efficacy of the form.
07
Test the form with a sample group to identify any issues or improvements needed.
08
Collect feedback and suggestions from the participants to further enhance the form's efficacy.
09
Revise and refine the form based on the feedback received.
10
Train and educate the users on how to properly fill out the form.
11
Regularly review and update the form as necessary to reflect changing evaluation requirements.
Who needs study evaluating form efficacy?
01
Researchers conducting studies
02
Educational institutions evaluating course effectiveness
03
Healthcare organizations assessing treatment outcomes
04
Government agencies monitoring program effectiveness
05
Organizations conducting performance evaluations
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I send study evaluating form efficacy for eSignature?
When you're ready to share your study evaluating form efficacy, you can send it to other people and get the eSigned document back just as quickly. Share your PDF by email, fax, text message, or USPS mail. You can also notarize your PDF on the web. You don't have to leave your account to do this.
How do I fill out the study evaluating form efficacy form on my smartphone?
You can easily create and fill out legal forms with the help of the pdfFiller mobile app. Complete and sign study evaluating form efficacy and other documents on your mobile device using the application. Visit pdfFiller’s webpage to learn more about the functionalities of the PDF editor.
Can I edit study evaluating form efficacy on an Android device?
Yes, you can. With the pdfFiller mobile app for Android, you can edit, sign, and share study evaluating form efficacy on your mobile device from any location; only an internet connection is needed. Get the app and start to streamline your document workflow from anywhere.
What is study evaluating form efficacy?
A study evaluating form efficacy assesses the effectiveness of a specific intervention, treatment, or program based on predefined criteria.
Who is required to file study evaluating form efficacy?
Researchers, institutions, or organizations conducting studies that evaluate the efficacy of treatments or interventions are required to file this form.
How to fill out study evaluating form efficacy?
To fill out the form, one must provide details such as study objectives, methodology, participant demographics, outcomes measured, and data analysis methods.
What is the purpose of study evaluating form efficacy?
The purpose is to gather systematic data on the effectiveness of treatments or programs, ensuring accountability and guiding evidence-based practices.
What information must be reported on study evaluating form efficacy?
Information such as study design, intervention details, population characteristics, results, and conclusions must be reported.
Fill out your study evaluating form efficacy online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Study Evaluating Form Efficacy is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















