Get the free Clinical trial of comparing the eect of ammonium lactate 14% and IPL
Show details
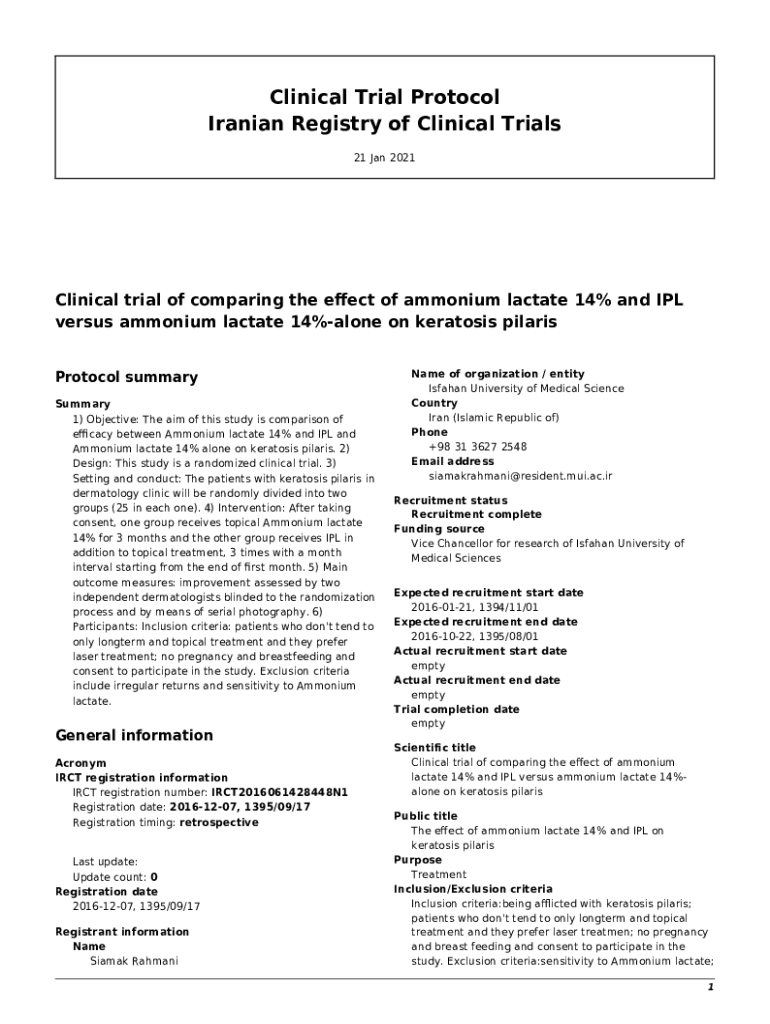
Clinical Trial Protocol
Iranian Registry of Clinical Trials
21 Jan 2021Clinical trial of comparing the elect of ammonium lactate 14% and ILL
versus ammonium lactate 14%alone on keratitis Polaris
Protocol
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign clinical trial of comparing

Edit your clinical trial of comparing form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your clinical trial of comparing form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing clinical trial of comparing online
Follow the guidelines below to use a professional PDF editor:
1
Check your account. If you don't have a profile yet, click Start Free Trial and sign up for one.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit clinical trial of comparing. Replace text, adding objects, rearranging pages, and more. Then select the Documents tab to combine, divide, lock or unlock the file.
4
Save your file. Choose it from the list of records. Then, shift the pointer to the right toolbar and select one of the several exporting methods: save it in multiple formats, download it as a PDF, email it, or save it to the cloud.
With pdfFiller, it's always easy to work with documents. Try it out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out clinical trial of comparing

How to fill out clinical trial of comparing
01
Identify the objective of the clinical trial: determine what specific treatments or interventions are being compared and what outcomes you are looking to measure.
02
Design the study: choose the study design that is most appropriate for your research question. This could include randomized controlled trials, non-randomized controlled trials, or observational studies.
03
Determine the sample size: calculate the number of participants needed to ensure statistical power and validity of the results.
04
Recruit participants: develop a recruitment strategy to identify and enroll eligible participants in the study.
05
Obtain informed consent: ensure that all participants understand the purpose, procedures, potential risks, and benefits of the clinical trial, and obtain their voluntary consent to participate.
06
Randomize and assign participants: use randomization techniques to assign participants to different treatment groups.
07
Implement the interventions: administer the treatments or interventions to the respective groups according to the study protocol.
08
Collect data: systematically gather data on relevant variables and outcomes for each participant.
09
Analyze the data: use appropriate statistical methods to analyze the collected data and assess the effectiveness or superiority of the treatments or interventions being compared.
10
Interpret the results: draw conclusions based on the analysis of the data and determine the implications for clinical practice or further research.
11
Communicate findings: disseminate the findings through publications, presentations, or other means to contribute to the existing body of knowledge on the topic.
Who needs clinical trial of comparing?
01
Researchers conducting clinical trials
02
Medical professionals seeking to evaluate the effectiveness of different treatments or interventions
03
Pharmaceutical companies testing new drugs or therapies
04
Regulatory authorities responsible for approving new medical interventions
05
Healthcare providers looking for evidence-based practice guidelines
06
Patients and their caregivers who want to make informed decisions about their treatment options
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I execute clinical trial of comparing online?
Filling out and eSigning clinical trial of comparing is now simple. The solution allows you to change and reorganize PDF text, add fillable fields, and eSign the document. Start a free trial of pdfFiller, the best document editing solution.
How do I make changes in clinical trial of comparing?
pdfFiller allows you to edit not only the content of your files, but also the quantity and sequence of the pages. Upload your clinical trial of comparing to the editor and make adjustments in a matter of seconds. Text in PDFs may be blacked out, typed in, and erased using the editor. You may also include photos, sticky notes, and text boxes, among other things.
How do I fill out the clinical trial of comparing form on my smartphone?
The pdfFiller mobile app makes it simple to design and fill out legal paperwork. Complete and sign clinical trial of comparing and other papers using the app. Visit pdfFiller's website to learn more about the PDF editor's features.
What is clinical trial of comparing?
A clinical trial of comparing is a study in which two or more interventions are tested against each other to determine which one is more effective or has fewer side effects.
Who is required to file clinical trial of comparing?
Researchers or sponsors conducting the clinical trial are required to file documentation for the clinical trial of comparing.
How to fill out clinical trial of comparing?
To fill out the clinical trial of comparing, you must provide specific details about the study design, interventions, participant eligibility, and methodologies according to regulatory guidelines.
What is the purpose of clinical trial of comparing?
The purpose of a clinical trial of comparing is to find out if one treatment is better than another in terms of efficacy and safety, thus helping to inform clinical decisions.
What information must be reported on clinical trial of comparing?
Information that must be reported includes study objectives, methodology, participant demographics, outcome measures, and results.
Fill out your clinical trial of comparing online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Clinical Trial Of Comparing is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















