Get the free Licensing Scheme for Drug Dependent Persons Treatment and ...
Show details
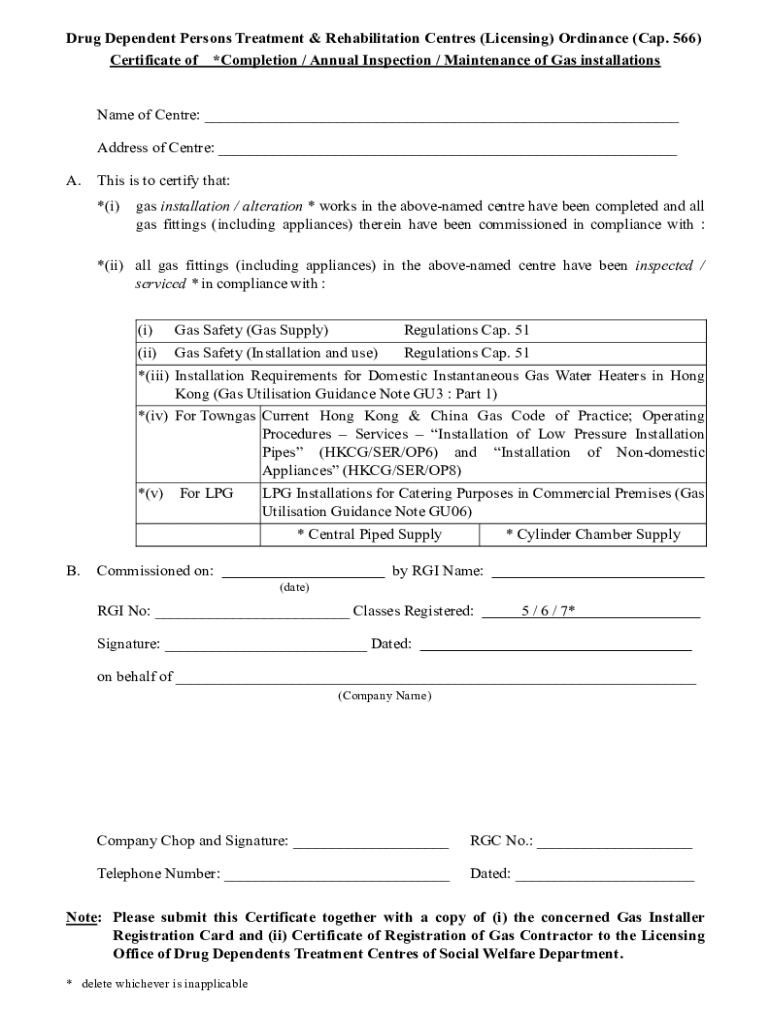
Drug Dependent Persons Treatment & Rehabilitation Centers (Licensing) Ordinance (Cap. 566) Certificate of *Completion / Annual Inspection / Maintenance of Gas installationsName of Center: Address
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign licensing scheme for drug

Edit your licensing scheme for drug form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your licensing scheme for drug form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit licensing scheme for drug online
Use the instructions below to start using our professional PDF editor:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Upload a file. Select Add New on your Dashboard and upload a file from your device or import it from the cloud, online, or internal mail. Then click Edit.
3
Edit licensing scheme for drug. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Save your file. Choose it from the list of records. Then, shift the pointer to the right toolbar and select one of the several exporting methods: save it in multiple formats, download it as a PDF, email it, or save it to the cloud.
Dealing with documents is always simple with pdfFiller.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out licensing scheme for drug

How to fill out licensing scheme for drug
01
To fill out the licensing scheme for drugs, follow these steps:
02
Start by obtaining the necessary application form for drug licensing from the appropriate regulatory authority.
03
Provide all the required information about the drug, including its name, purpose, composition, and intended use.
04
Include the necessary documentation, such as clinical trial results, safety data, and manufacturing details.
05
Pay the required fees for the licensing process.
06
Submit the completed application form along with all supporting documents to the regulatory authority.
07
Await the review and evaluation process by the regulatory authority, which may include inspections and assessments.
08
Address any additional information or queries requested by the regulatory authority during the evaluation process.
09
Once the licensing scheme for the drug is approved, comply with any post-approval requirements or conditions set by the regulatory authority.
10
Renew the drug license periodically as per the regulations and guidelines.
11
Remember to consult the specific regulatory authority's guidelines and requirements for accurate and up-to-date instructions.
Who needs licensing scheme for drug?
01
A licensing scheme for drugs is needed by various stakeholders in the pharmaceutical industry, including:
02
- Pharmaceutical manufacturers who want to legally produce and market drugs in compliance with regulatory standards.
03
- Healthcare professionals who prescribe and administer drugs, ensuring the medications meet safety and quality standards.
04
- Regulatory authorities who establish and enforce regulations to safeguard public health and ensure the efficacy and safety of drugs.
05
- Patients and consumers who rely on licensed drugs for their medical treatment, ensuring they have access to safe and effective medications.
06
- Researchers and scientists who require licensed drugs for their studies and clinical trials to evaluate their effectiveness and potential side effects.
07
Overall, a licensing scheme for drugs is essential to ensure transparency, quality control, public safety, and trust within the pharmaceutical industry.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
Where do I find licensing scheme for drug?
The premium subscription for pdfFiller provides you with access to an extensive library of fillable forms (over 25M fillable templates) that you can download, fill out, print, and sign. You won’t have any trouble finding state-specific licensing scheme for drug and other forms in the library. Find the template you need and customize it using advanced editing functionalities.
How do I execute licensing scheme for drug online?
pdfFiller has made it simple to fill out and eSign licensing scheme for drug. The application has capabilities that allow you to modify and rearrange PDF content, add fillable fields, and eSign the document. Begin a free trial to discover all of the features of pdfFiller, the best document editing solution.
Can I create an electronic signature for the licensing scheme for drug in Chrome?
You can. With pdfFiller, you get a strong e-signature solution built right into your Chrome browser. Using our addon, you may produce a legally enforceable eSignature by typing, sketching, or photographing it. Choose your preferred method and eSign in minutes.
What is licensing scheme for drug?
A licensing scheme for drugs is a regulatory framework that governs the approval, distribution, and use of pharmaceutical products to ensure safety, efficacy, and quality.
Who is required to file licensing scheme for drug?
Manufacturers and distributors of pharmaceutical products are typically required to file a licensing scheme for drugs.
How to fill out licensing scheme for drug?
To fill out a licensing scheme for a drug, applicants must provide detailed information about the drug's formulation, manufacturing process, clinical data, labeling, and compliance with regulatory requirements.
What is the purpose of licensing scheme for drug?
The purpose of a licensing scheme for drugs is to ensure that medications are safe for use, effective for their intended purposes, and manufactured according to accepted standards.
What information must be reported on licensing scheme for drug?
Information that must be reported typically includes drug composition, manufacturing process, evidence of clinical trials, safety data, and proposed labeling.
Fill out your licensing scheme for drug online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Licensing Scheme For Drug is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.