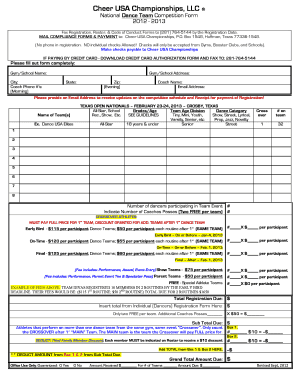
Get the free Case Report Form (CRF) (10 June 2010) - EUTOS for CML - leukemia-net
Show details
EUROS FOR CML Registry European Treatment and Outcome Study for Chronic Myeloid Leukemia (EUROS for CML) European CML Registry population-based section Case Report Form (CRF) (10 June 2010) When filling
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign case report form crf

Edit your case report form crf form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your case report form crf form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing case report form crf online
To use our professional PDF editor, follow these steps:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit case report form crf. Add and change text, add new objects, move pages, add watermarks and page numbers, and more. Then click Done when you're done editing and go to the Documents tab to merge or split the file. If you want to lock or unlock the file, click the lock or unlock button.
4
Save your file. Select it in the list of your records. Then, move the cursor to the right toolbar and choose one of the available exporting methods: save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud.
pdfFiller makes working with documents easier than you could ever imagine. Try it for yourself by creating an account!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out case report form crf

How to fill out a case report form (CRF):
01
Start by reviewing the specific instructions or guidelines provided for completing the CRF. This may include information on what sections need to be completed, any specific formatting requirements, and how to handle certain types of data.
02
Begin by entering the necessary identifying information at the top of the form, such as the patient's name, medical record number, and study identification number. Make sure to double-check the accuracy of this information.
03
Proceed to fill in the demographic details of the patient, including age, gender, and medical history. If applicable, also provide information about the patient's ethnicity, race, and relevant lifestyle factors.
04
Document the patient's medical condition accurately and comprehensively. This may involve recording details about the diagnosis, disease severity, current symptoms, treatment history, and any medications or procedures undergone by the patient.
05
If the CRF includes sections for multiple study visits, ensure that you accurately and precisely record the information for each visit in a chronological order. This includes documenting any changes in the patient's condition, treatment modifications, adverse events, or concomitant medications.
06
Pay attention to any specific data formats required for certain sections, such as date formats or numerical values. Make sure to follow these instructions meticulously to maintain data integrity.
07
If there are any sections in the CRF that are not applicable to the patient or study, clearly mark them as "N/A" or "not applicable." This will help to avoid confusion and ensure that all required sections are appropriately addressed.
08
Review the completed CRF thoroughly for any errors, omissions, or inconsistencies. It is essential to maintain accuracy and quality in the reported data. If needed, consult with a healthcare professional or study coordinator to clarify any uncertainties.
Who needs a case report form (CRF)?
01
Clinical researchers conducting medical studies require CRFs to systematically collect and record data related to patients' medical conditions, treatments, and outcomes.
02
Pharmaceutical companies and regulatory authorities often mandate the use of CRFs for clinical trials to ensure standardized data collection and facilitate analysis and reporting.
03
Healthcare professionals involved in patient care may use CRFs to compile comprehensive medical records, especially in cases where detailed data is required or for documenting rare diseases.
Overall, CRFs play a crucial role in collecting accurate and standardized data for research, regulatory, and clinical purposes, allowing for evidence-based decision-making and further advancements in healthcare.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is case report form crf?
A case report form (CRF) is a document used in clinical research to collect data from study participants.
Who is required to file case report form crf?
The researchers or healthcare professionals conducting the clinical study are required to file the case report form (CRF).
How to fill out case report form crf?
The case report form (CRF) can be filled out by entering data either manually or electronically, following the instructions provided by the study protocol.
What is the purpose of case report form crf?
The purpose of the case report form (CRF) is to collect accurate and complete data on study participants in a standardized format.
What information must be reported on case report form crf?
The case report form (CRF) typically includes information on demographics, medical history, treatment received, and study outcomes.
How do I edit case report form crf straight from my smartphone?
The best way to make changes to documents on a mobile device is to use pdfFiller's apps for iOS and Android. You may get them from the Apple Store and Google Play. Learn more about the apps here. To start editing case report form crf, you need to install and log in to the app.
How do I fill out the case report form crf form on my smartphone?
You can easily create and fill out legal forms with the help of the pdfFiller mobile app. Complete and sign case report form crf and other documents on your mobile device using the application. Visit pdfFiller’s webpage to learn more about the functionalities of the PDF editor.
Can I edit case report form crf on an iOS device?
You certainly can. You can quickly edit, distribute, and sign case report form crf on your iOS device with the pdfFiller mobile app. Purchase it from the Apple Store and install it in seconds. The program is free, but in order to purchase a subscription or activate a free trial, you must first establish an account.
Fill out your case report form crf online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Case Report Form Crf is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.