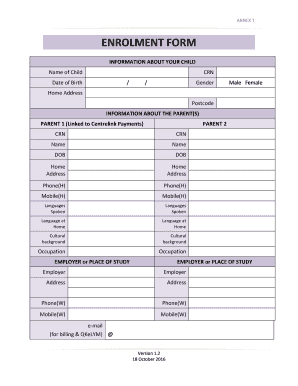
Get the free vaccine adverse event reporting system form - wcchd
Show details
WEBSITE: www.vaers.hhs.gov E-MAIL: info veers.org FAX: 1-877-721-0366 VACCINE ADVERSE EVENT REPORTING SYSTEM 24 Hour Toll-Free Information 1-800-822-7967 PATIENT IDENTITY KEPT CONFIDENTIAL Patient
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign vaccine adverse event reporting

Edit your vaccine adverse event reporting form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your vaccine adverse event reporting form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit vaccine adverse event reporting online
Use the instructions below to start using our professional PDF editor:
1
Create an account. Begin by choosing Start Free Trial and, if you are a new user, establish a profile.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit vaccine adverse event reporting. Replace text, adding objects, rearranging pages, and more. Then select the Documents tab to combine, divide, lock or unlock the file.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
It's easier to work with documents with pdfFiller than you can have ever thought. You can sign up for an account to see for yourself.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out vaccine adverse event reporting

How to fill out vaccine adverse event reporting:
01
Begin by gathering all relevant information about the vaccine recipient, such as their name, age, and contact details.
02
Provide details about the vaccine, including the name, manufacturer, lot number, and date of administration.
03
Describe the adverse event experienced by the individual, specifying the symptoms, their severity, and any medical interventions required.
04
Include any relevant medical history or underlying health conditions that may have contributed to the adverse event.
05
If applicable, share information about any concomitant medications or vaccines taken by the recipient.
06
Include the contact information of the healthcare professional who administered the vaccine, as well as their professional credentials.
07
Submit the completed vaccine adverse event reporting form through the designated reporting channels, such as the national or regional adverse event reporting system.
Who needs vaccine adverse event reporting:
01
Healthcare professionals, including doctors, nurses, and pharmacists, who administer vaccines need to report any adverse events they come across.
02
Pharmaceutical companies and vaccine manufacturers need to collect and analyze adverse event reports to ensure the safety and efficacy of their products.
03
Regulatory authorities, such as the Food and Drug Administration (FDA) or the European Medicines Agency (EMA), require vaccine adverse event reporting to monitor the safety and quality of vaccines.
04
Researchers and public health experts rely on adverse event reporting data to identify patterns, assess vaccine safety, and make informed decisions regarding immunization programs.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I manage my vaccine adverse event reporting directly from Gmail?
In your inbox, you may use pdfFiller's add-on for Gmail to generate, modify, fill out, and eSign your vaccine adverse event reporting and any other papers you receive, all without leaving the program. Install pdfFiller for Gmail from the Google Workspace Marketplace by visiting this link. Take away the need for time-consuming procedures and handle your papers and eSignatures with ease.
Where do I find vaccine adverse event reporting?
The premium subscription for pdfFiller provides you with access to an extensive library of fillable forms (over 25M fillable templates) that you can download, fill out, print, and sign. You won’t have any trouble finding state-specific vaccine adverse event reporting and other forms in the library. Find the template you need and customize it using advanced editing functionalities.
How do I complete vaccine adverse event reporting on an Android device?
Use the pdfFiller app for Android to finish your vaccine adverse event reporting. The application lets you do all the things you need to do with documents, like add, edit, and remove text, sign, annotate, and more. There is nothing else you need except your smartphone and an internet connection to do this.
What is vaccine adverse event reporting?
Vaccine adverse event reporting is the process of documenting and reporting any negative or unexpected side effects or events that occur after administering a vaccine.
Who is required to file vaccine adverse event reporting?
Healthcare providers, manufacturers, and distributors are required to file vaccine adverse event reporting.
How to fill out vaccine adverse event reporting?
To fill out vaccine adverse event reporting, healthcare providers should collect relevant information about the patient, vaccine details, and the adverse event. This information can then be submitted through the designated reporting system.
What is the purpose of vaccine adverse event reporting?
The purpose of vaccine adverse event reporting is to monitor and track any potential safety concerns related to vaccines. It helps regulatory authorities, healthcare providers, and manufacturers to ensure the safety of vaccines.
What information must be reported on vaccine adverse event reporting?
Vaccine adverse event reporting should include information such as patient demographics, vaccine details (e.g., brand, lot number), the adverse event description, and any relevant medical history.
Fill out your vaccine adverse event reporting online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Vaccine Adverse Event Reporting is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.