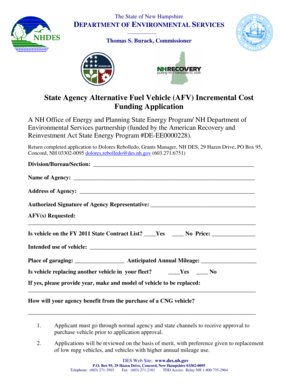
Get the free Phase 4 Clinical Reflection/Narrative (WTS 1-10) Directions
Show details
Phase 4 Clinical Reflection/Narrative (WAS 110) Directions
(From student guide)
Divide the Clinical Reflection/Narrative into a series of subsections. In each subsection, address
one WAS and answer
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign phase 4 clinical reflectionnarrative

Edit your phase 4 clinical reflectionnarrative form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your phase 4 clinical reflectionnarrative form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit phase 4 clinical reflectionnarrative online
Use the instructions below to start using our professional PDF editor:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit phase 4 clinical reflectionnarrative. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Get your file. Select the name of your file in the docs list and choose your preferred exporting method. You can download it as a PDF, save it in another format, send it by email, or transfer it to the cloud.
pdfFiller makes dealing with documents a breeze. Create an account to find out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out phase 4 clinical reflectionnarrative

How to fill out phase 4 clinical reflectionnarrative
01
To fill out a phase 4 clinical reflection narrative, follow these steps:
02
Start by reviewing the specific guidelines or requirements provided by the regulatory body or study sponsor for the phase 4 clinical trial.
03
Gather all the necessary information and data related to the trial, including patient reports, laboratory results, and any other relevant documentation.
04
Begin the narrative by providing a concise overview of the trial, including its purpose, objectives, and methodology.
05
Describe the patient population that was involved in the trial, providing details such as the number of participants, their demographic characteristics, and any specific inclusion or exclusion criteria.
06
Explain the interventions or treatments that were administered to the participants, along with any associated protocols or guidelines followed.
07
Discuss the outcomes or results observed during the trial, highlighting any significant findings or trends.
08
Reflect on the implications of the trial's results and discuss how they contribute to the existing body of knowledge or future research.
09
Conclude the narrative by summarizing the key points and stating any recommendations or conclusions drawn from the trial.
10
Proofread and edit the narrative for clarity, coherence, and accuracy.
11
Follow any additional formatting or submission guidelines provided, and submit the filled-out phase 4 clinical reflection narrative as required.
Who needs phase 4 clinical reflectionnarrative?
01
Phase 4 clinical reflection narratives are typically required by researchers, healthcare professionals, and regulatory bodies involved in post-marketing surveillance and monitoring of drugs or medical devices.
02
Pharmaceutical companies, regulatory agencies, and academic institutions may also request phase 4 clinical reflection narratives as part of the ongoing evaluation and assessment of the safety and effectiveness of a medical product.
03
Additionally, healthcare providers who have participated in phase 4 clinical trials may need to fill out a reflection narrative to document their experience and contribute to the collective knowledge in the field.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I complete phase 4 clinical reflectionnarrative online?
Filling out and eSigning phase 4 clinical reflectionnarrative is now simple. The solution allows you to change and reorganize PDF text, add fillable fields, and eSign the document. Start a free trial of pdfFiller, the best document editing solution.
How do I edit phase 4 clinical reflectionnarrative on an Android device?
The pdfFiller app for Android allows you to edit PDF files like phase 4 clinical reflectionnarrative. Mobile document editing, signing, and sending. Install the app to ease document management anywhere.
How do I fill out phase 4 clinical reflectionnarrative on an Android device?
Use the pdfFiller mobile app to complete your phase 4 clinical reflectionnarrative on an Android device. The application makes it possible to perform all needed document management manipulations, like adding, editing, and removing text, signing, annotating, and more. All you need is your smartphone and an internet connection.
What is phase 4 clinical reflection narrative?
Phase 4 clinical reflection narrative refers to a comprehensive report that summarizes the findings and experiences gathered during the post-marketing surveillance of a drug or treatment after it has been approved for public use.
Who is required to file phase 4 clinical reflection narrative?
Pharmaceutical companies and sponsors of clinical trials are typically required to file phase 4 clinical reflection narratives as part of their obligations to regulatory authorities.
How to fill out phase 4 clinical reflection narrative?
To fill out a phase 4 clinical reflection narrative, one must gather relevant clinical data, summarize key findings, document the implications of the results, and adhere to the required format and guidelines set by regulatory agencies.
What is the purpose of phase 4 clinical reflection narrative?
The purpose of a phase 4 clinical reflection narrative is to provide insights into the long-term effects, safety, and efficacy of a drug after it has been released to the market, ensuring ongoing assessment of its benefits and risks.
What information must be reported on phase 4 clinical reflection narrative?
Information that must be reported includes adverse events, efficacy data, demographic information of trial participants, insights on drug interactions, and any other relevant clinical findings.
Fill out your phase 4 clinical reflectionnarrative online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Phase 4 Clinical Reflectionnarrative is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.