Get the free A prospective study of child adjustment to cardiac ...
Show details
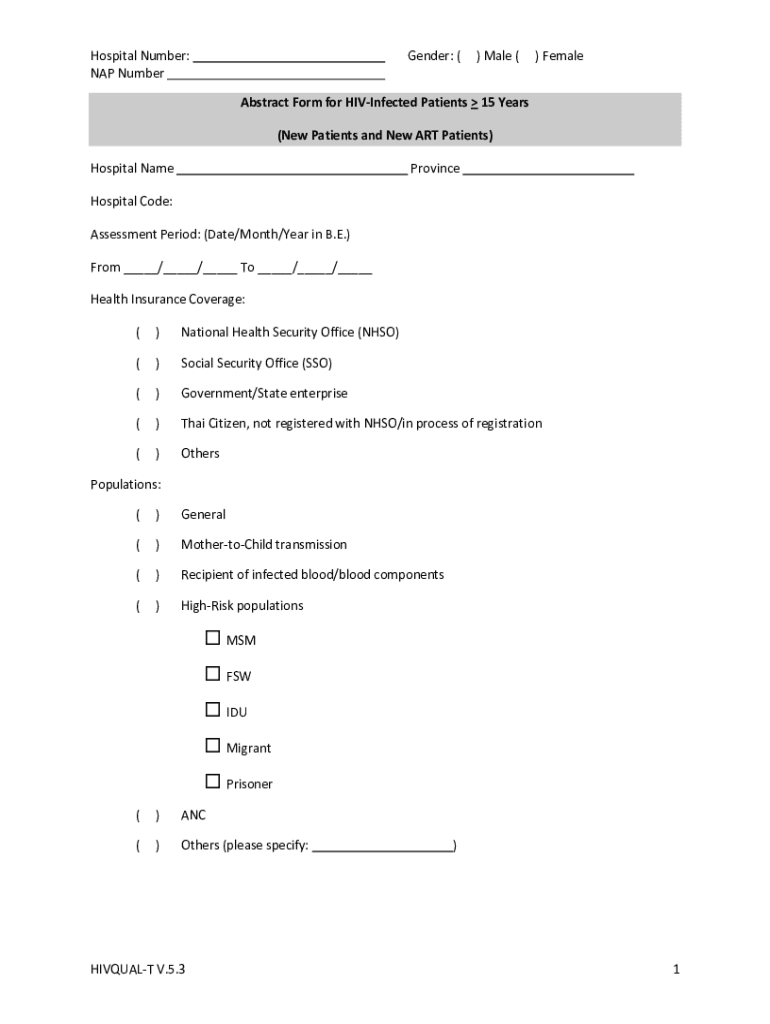
Hospital Number: NAP NumberGender: () Male () FemaleAbstract Form for Disinfected Patients 15 Years (New Patients and New ART Patients) Hospital NameProvinceHospital Code: Assessment Period: (Date/Month/Year
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign a prospective study of

Edit your a prospective study of form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your a prospective study of form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit a prospective study of online
Use the instructions below to start using our professional PDF editor:
1
Create an account. Begin by choosing Start Free Trial and, if you are a new user, establish a profile.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit a prospective study of. Rearrange and rotate pages, add new and changed texts, add new objects, and use other useful tools. When you're done, click Done. You can use the Documents tab to merge, split, lock, or unlock your files.
4
Get your file. Select the name of your file in the docs list and choose your preferred exporting method. You can download it as a PDF, save it in another format, send it by email, or transfer it to the cloud.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out a prospective study of

How to fill out a prospective study of
01
To fill out a prospective study, follow these steps:
02
Determine the objective of the study: Clearly define the research question or hypothesis you want to address with the prospective study.
03
Design the study protocol: Develop a detailed plan that outlines the study design, participant selection criteria, data collection methods, and follow-up procedures.
04
Obtain ethical approval: Ensure that your study design and procedures comply with ethical guidelines and obtain approval from an institutional review board or ethics committee.
05
Recruit participants: Identify and recruit a suitable sample of participants who meet your selection criteria and obtain their informed consent to participate in the study.
06
Collect baseline data: Gather relevant baseline information from the participants before any intervention or exposure occurs.
07
Implement interventions/exposure: Carry out the planned interventions or expose the participants to the defined factors of interest.
08
Conduct follow-up assessments: Regularly assess and collect data from the participants at specified time points to track any outcomes or changes of interest.
09
Analyze and interpret data: Analyze the collected data using appropriate statistical methods and interpret the findings.
10
Draw conclusions and report findings: Conclude the study based on the analysis results and publish or present the findings in a scientific format.
11
Consider limitations and future directions: Reflect on the study's limitations and suggest potential areas for future research.
12
Remember to adhere to good research practices, maintain data integrity, and ensure participant confidentiality throughout the study.
Who needs a prospective study of?
01
Prospective studies are valuable for various individuals and groups, including:
02
- Researchers: Prospective studies allow researchers to investigate the cause-effect relationship between variables over time, providing valuable insights into long-term outcomes.
03
- Medical professionals: Prospective studies help medical professionals assess the effectiveness and safety of new treatments or interventions to guide clinical decision-making.
04
- Public health practitioners: Prospective studies contribute to a better understanding of disease prevention, risk factors, and the development of public health policies.
05
- Pharmaceutical companies: Prospective studies assist pharmaceutical companies in evaluating the long-term effects and benefits of their products.
06
- Patients: Prospective studies can directly benefit patients by improving healthcare practices, identifying potential risk factors, and enhancing treatment outcomes.
07
Overall, anyone involved in research, healthcare, public health, or the pharmaceutical industry can benefit from and utilize the findings of prospective studies.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I modify my a prospective study of in Gmail?
You may use pdfFiller's Gmail add-on to change, fill out, and eSign your a prospective study of as well as other documents directly in your inbox by using the pdfFiller add-on for Gmail. pdfFiller for Gmail may be found on the Google Workspace Marketplace. Use the time you would have spent dealing with your papers and eSignatures for more vital tasks instead.
Can I create an electronic signature for signing my a prospective study of in Gmail?
You may quickly make your eSignature using pdfFiller and then eSign your a prospective study of right from your mailbox using pdfFiller's Gmail add-on. Please keep in mind that in order to preserve your signatures and signed papers, you must first create an account.
Can I edit a prospective study of on an Android device?
With the pdfFiller mobile app for Android, you may make modifications to PDF files such as a prospective study of. Documents may be edited, signed, and sent directly from your mobile device. Install the app and you'll be able to manage your documents from anywhere.
What is a prospective study of?
A prospective study is an investigation that follows participants over time to observe outcomes or incidents based on certain exposures or interventions.
Who is required to file a prospective study of?
Researchers or institutions conducting studies that involve human participants are typically required to file a prospective study.
How to fill out a prospective study of?
Filling out a prospective study usually involves detailing the study design, objectives, participant criteria, methods of data collection, and analysis plans in a formal submission to an ethical board or regulatory agency.
What is the purpose of a prospective study of?
The purpose of a prospective study is to assess the relationship between potential risk factors and outcomes by collecting data moving forward in time.
What information must be reported on a prospective study of?
Information that must be reported includes study objectives, methodology, participant criteria, expected outcomes, duration, and any ethical considerations.
Fill out your a prospective study of online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

A Prospective Study Of is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















