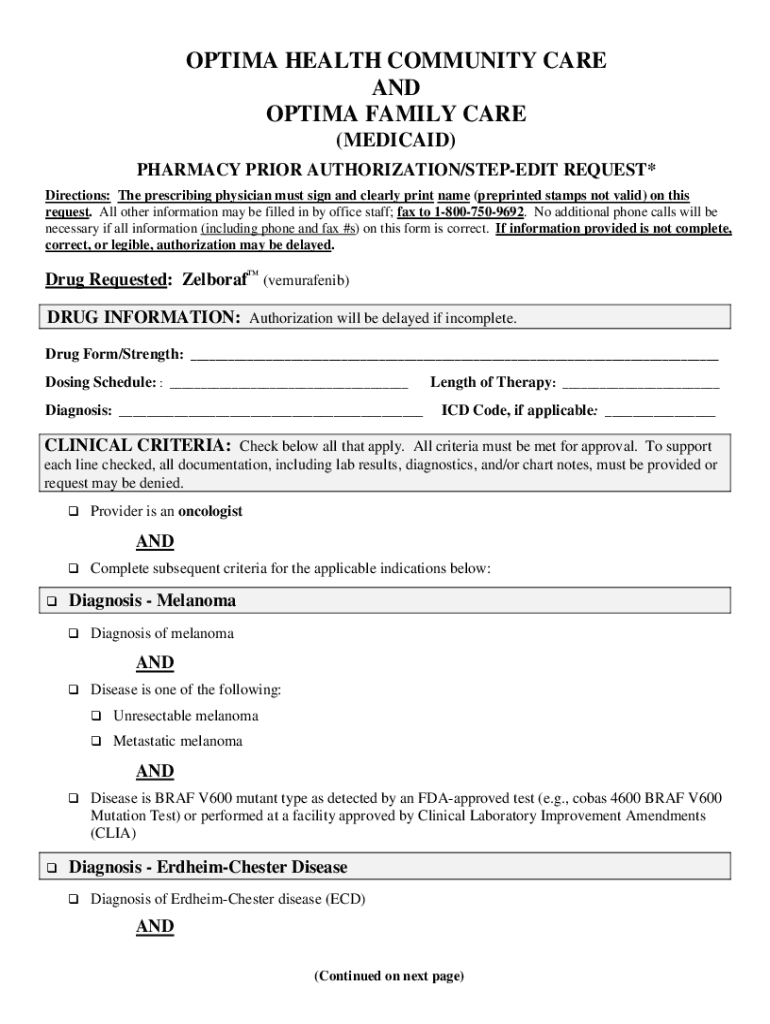
Get the free Drug Requested: (vemurafenib)
Show details
OPTIMA HEALTH COMMUNITY CARE
AND
OPTIMA FAMILY CARE
(MEDICAID)
PHARMACY PRIOR AUTHORIZATION/STEPPED REQUEST*
Directions: The prescribing physician must sign and clearly print name (preprinted stamps
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign drug requested vemurafenib

Edit your drug requested vemurafenib form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your drug requested vemurafenib form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit drug requested vemurafenib online
To use the professional PDF editor, follow these steps below:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Upload a file. Select Add New on your Dashboard and upload a file from your device or import it from the cloud, online, or internal mail. Then click Edit.
3
Edit drug requested vemurafenib. Add and replace text, insert new objects, rearrange pages, add watermarks and page numbers, and more. Click Done when you are finished editing and go to the Documents tab to merge, split, lock or unlock the file.
4
Get your file. When you find your file in the docs list, click on its name and choose how you want to save it. To get the PDF, you can save it, send an email with it, or move it to the cloud.
With pdfFiller, it's always easy to deal with documents.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out drug requested vemurafenib

How to fill out drug requested vemurafenib
01
To fill out drug requested vemurafenib, follow these steps:
02
Obtain the prescription for vemurafenib from a licensed healthcare professional.
03
Visit a pharmacy that stocks vemurafenib.
04
Present the prescription to the pharmacist.
05
Provide any necessary personal and insurance information to the pharmacist.
06
The pharmacist will dispense the appropriate amount of vemurafenib.
07
Follow the instructions provided by the pharmacist and the medication guide.
08
Take vemurafenib as directed by your healthcare provider.
09
Contact your healthcare provider if you have any questions or concerns.
10
Store vemurafenib as instructed, away from heat, light, and moisture.
11
Dispose of any unused or expired vemurafenib properly, according to local regulations.
Who needs drug requested vemurafenib?
01
Vemurafenib is a medication used to treat certain types of skin cancer, specifically melanoma, which is caused by a mutation in the BRAF gene.
02
Patients who have been diagnosed with unresectable or metastatic melanoma with a BRAF V600 mutation may benefit from vemurafenib.
03
It is important to note that vemurafenib should only be used under the supervision of a healthcare professional who has experience in the treatment of melanoma.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I manage my drug requested vemurafenib directly from Gmail?
drug requested vemurafenib and other documents can be changed, filled out, and signed right in your Gmail inbox. You can use pdfFiller's add-on to do this, as well as other things. When you go to Google Workspace, you can find pdfFiller for Gmail. You should use the time you spend dealing with your documents and eSignatures for more important things, like going to the gym or going to the dentist.
How do I complete drug requested vemurafenib on an iOS device?
Install the pdfFiller iOS app. Log in or create an account to access the solution's editing features. Open your drug requested vemurafenib by uploading it from your device or online storage. After filling in all relevant fields and eSigning if required, you may save or distribute the document.
Can I edit drug requested vemurafenib on an Android device?
You can make any changes to PDF files, such as drug requested vemurafenib, with the help of the pdfFiller mobile app for Android. Edit, sign, and send documents right from your mobile device. Install the app and streamline your document management wherever you are.
What is drug requested vemurafenib?
Vemurafenib is a targeted cancer therapy used primarily for the treatment of melanoma with a specific BRAF mutation.
Who is required to file drug requested vemurafenib?
Healthcare providers, pharmaceutical companies, and research institutions that are involved in the use or investigation of vemurafenib are required to file.
How to fill out drug requested vemurafenib?
Fill out the drug request form by providing patient details, dosage information, treatment duration, and relevant medical history.
What is the purpose of drug requested vemurafenib?
The purpose is to obtain authorization for the use of vemurafenib in eligible patients and to ensure proper monitoring of its safety and efficacy.
What information must be reported on drug requested vemurafenib?
Required information includes patient identification, BRAF mutation status, prior treatments, dosage proposed, and any adverse reactions.
Fill out your drug requested vemurafenib online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Drug Requested Vemurafenib is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















