Get the free Regulatory oversight of biochemical pesticides by the U.S. ... - PubMed
Show details
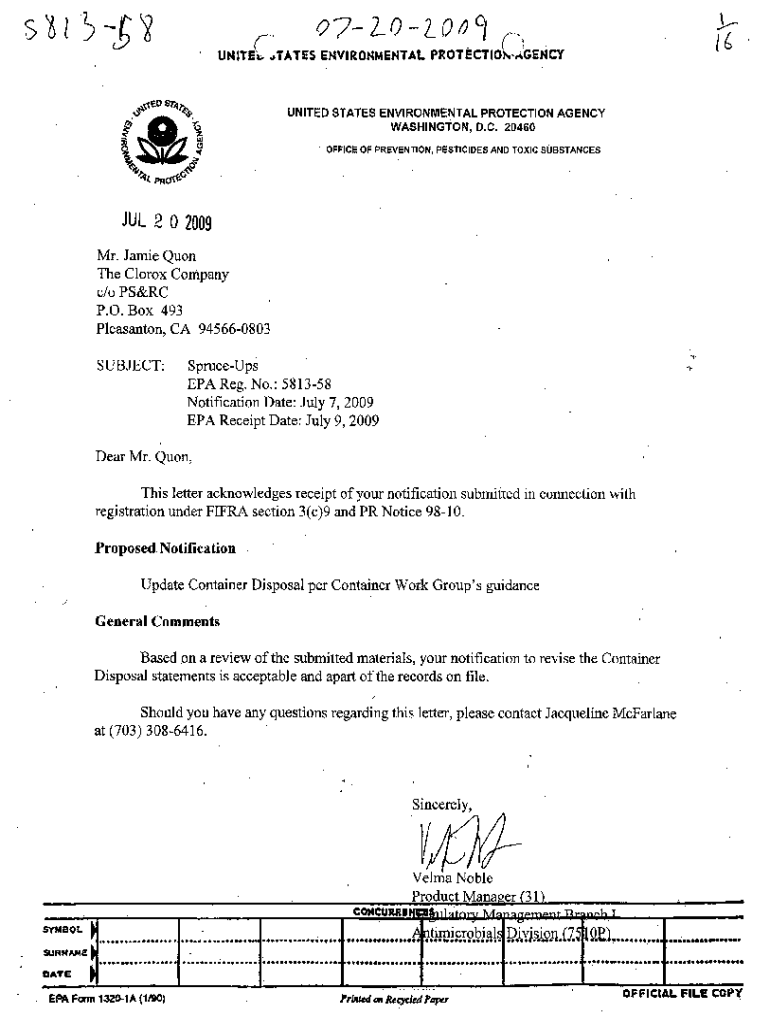
“, 07LOLOO ('1. UNITECTATES ENVIRONMENTAL PROTECTION “AGENCY UNITED STATES ENVIRONMENTAL PROTECTION AGENCY WASHINGTON, D.C. 20460. OFFICE OF PREVENTION, PESTICIDES AND TOXIC SUBSTANCES 2 0 2009
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign regulatory oversight of biochemical

Edit your regulatory oversight of biochemical form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your regulatory oversight of biochemical form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing regulatory oversight of biochemical online
To use the professional PDF editor, follow these steps below:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit regulatory oversight of biochemical. Text may be added and replaced, new objects can be included, pages can be rearranged, watermarks and page numbers can be added, and so on. When you're done editing, click Done and then go to the Documents tab to combine, divide, lock, or unlock the file.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
pdfFiller makes working with documents easier than you could ever imagine. Register for an account and see for yourself!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out regulatory oversight of biochemical

How to fill out regulatory oversight of biochemical
01
To fill out the regulatory oversight of biochemical, follow these steps:
02
Identify the specific regulatory requirements for biochemicals in your jurisdiction.
03
Understand the purpose and goals of the regulatory oversight.
04
Gather all the necessary information and documentation related to the biochemical product or process.
05
Assess the potential risks and hazards associated with the biochemical and determine appropriate safety measures.
06
Develop a comprehensive plan that includes steps for compliance with the regulatory requirements.
07
Implement the necessary controls and procedures to ensure adherence to the regulations.
08
Establish a system for monitoring and reporting any incidents or non-compliance.
09
Regularly review and update the regulatory oversight process to stay compliant with any changes in regulations.
10
Train and educate all relevant personnel to understand and follow the necessary procedures.
11
Maintain accurate records and documentation to demonstrate compliance with the regulatory oversight.
Who needs regulatory oversight of biochemical?
01
Various entities and individuals require regulatory oversight of biochemicals, including:
02
- Manufacturers and producers of biochemical products.
03
- Researchers and scientists working with biochemical substances.
04
- Government agencies responsible for ensuring public safety and environmental protection.
05
- Regulatory bodies and authorities overseeing the use and distribution of biochemicals.
06
- Health and safety organizations promoting workplace and consumer safety.
07
- Importers and exporters of biochemical products.
08
- Consumers and end-users of biochemical products who rely on their safety and quality.
09
- Healthcare providers who administer or prescribe biochemical-based treatments.
10
- Academic institutions and educational facilities offering programs in biochemistry.
11
- Non-profit organizations advocating for the responsible and ethical use of biochemicals.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
Where do I find regulatory oversight of biochemical?
The premium version of pdfFiller gives you access to a huge library of fillable forms (more than 25 million fillable templates). You can download, fill out, print, and sign them all. State-specific regulatory oversight of biochemical and other forms will be easy to find in the library. Find the template you need and use advanced editing tools to make it your own.
How do I edit regulatory oversight of biochemical online?
With pdfFiller, the editing process is straightforward. Open your regulatory oversight of biochemical in the editor, which is highly intuitive and easy to use. There, you’ll be able to blackout, redact, type, and erase text, add images, draw arrows and lines, place sticky notes and text boxes, and much more.
How do I fill out the regulatory oversight of biochemical form on my smartphone?
On your mobile device, use the pdfFiller mobile app to complete and sign regulatory oversight of biochemical. Visit our website (https://edit-pdf-ios-android.pdffiller.com/) to discover more about our mobile applications, the features you'll have access to, and how to get started.
What is regulatory oversight of biochemical?
Regulatory oversight of biochemical refers to the supervision and regulation of biochemical products and processes by governmental or authorized agencies to ensure compliance with safety, efficacy, and quality standards.
Who is required to file regulatory oversight of biochemical?
Companies, researchers, and manufacturers involved in the development, production, and distribution of biochemical products are typically required to file for regulatory oversight.
How to fill out regulatory oversight of biochemical?
To fill out regulatory oversight of biochemical, entities must complete the specific forms provided by the regulatory authority, providing detailed information on the product, its components, testing data, and compliance with relevant guidelines.
What is the purpose of regulatory oversight of biochemical?
The purpose of regulatory oversight of biochemical is to protect public health by ensuring that biochemical products are safe for use, effective in their intended applications, and that they meet established quality standards.
What information must be reported on regulatory oversight of biochemical?
Information that must be reported includes the product's composition, manufacturing processes, safety and efficacy data, labeling details, and any adverse effects encountered during testing.
Fill out your regulatory oversight of biochemical online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Regulatory Oversight Of Biochemical is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.




















