Get the free Interpretive Guidelines for Laboratories CMS
Show details
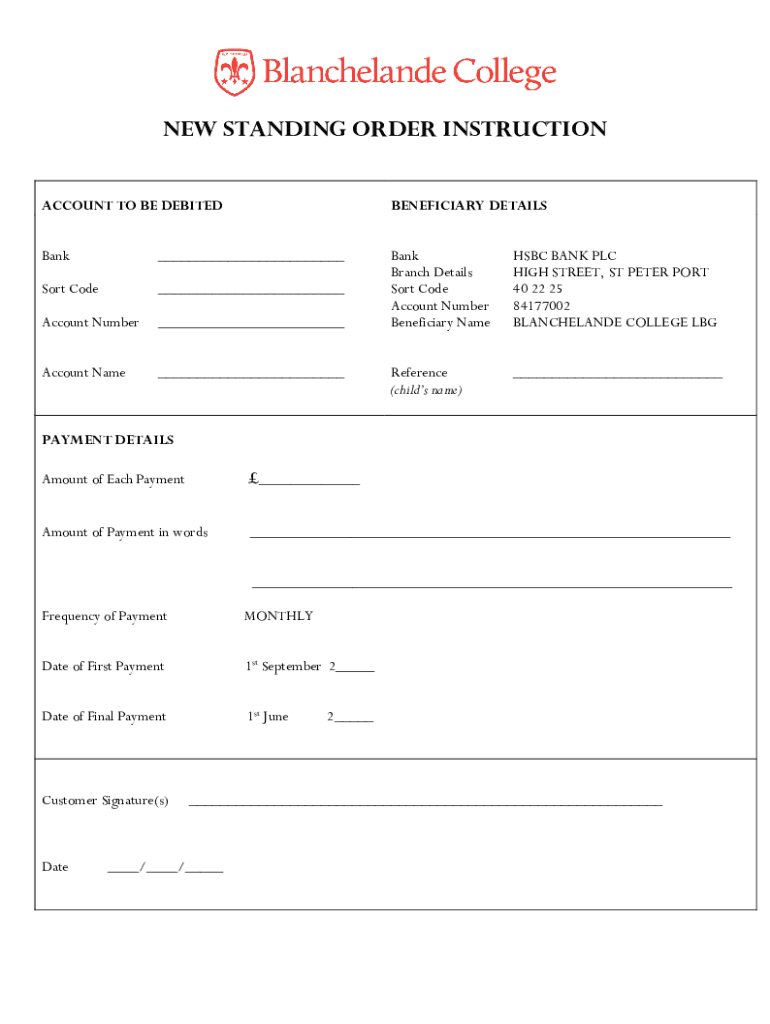
NEW STANDING ORDER INSTRUCTION ACCOUNT TO BE DEBITEDBENEFICIARY DETAILSBank Sort Code Account Number Bank Branch Details Sort Code Account Number Beneficiary Name HSBC BANK PLC HIGH STREET, ST PETER
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign interpretive guidelines for laboratories

Edit your interpretive guidelines for laboratories form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your interpretive guidelines for laboratories form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit interpretive guidelines for laboratories online
Follow the steps below to take advantage of the professional PDF editor:
1
Log in. Click Start Free Trial and create a profile if necessary.
2
Upload a file. Select Add New on your Dashboard and upload a file from your device or import it from the cloud, online, or internal mail. Then click Edit.
3
Edit interpretive guidelines for laboratories. Text may be added and replaced, new objects can be included, pages can be rearranged, watermarks and page numbers can be added, and so on. When you're done editing, click Done and then go to the Documents tab to combine, divide, lock, or unlock the file.
4
Get your file. Select the name of your file in the docs list and choose your preferred exporting method. You can download it as a PDF, save it in another format, send it by email, or transfer it to the cloud.
It's easier to work with documents with pdfFiller than you can have ever thought. Sign up for a free account to view.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out interpretive guidelines for laboratories

How to fill out interpretive guidelines for laboratories
01
To fill out interpretive guidelines for laboratories, follow these steps:
02
Gather all relevant information: Start by collecting all the necessary data and information about the laboratory and its tests. This includes the purpose of the guidelines, the types of tests conducted, and any specific requirements or standards that should be followed.
03
Define the structure: Decide on the layout and organization of the guidelines. This could be done in a point-by-point format, with each point addressing a specific aspect or test.
04
Write clear instructions: Clearly articulate each guideline, making sure it is easy to understand and follow. Use simple language and avoid jargon or technical terms whenever possible.
05
Include examples: Provide examples or case studies to illustrate the guidelines and make them more practical and applicable to real-world situations.
06
Review and revise: Proofread and review the guidelines for any errors or inconsistencies. Make necessary revisions to improve clarity and accuracy.
07
Seek expert input: If possible, consult with subject matter experts or experienced laboratory professionals to get their input and validate the guidelines.
08
Finalize and publish: Once the guidelines are complete and approved, finalize them and make them publicly available either through print or digital mediums.
Who needs interpretive guidelines for laboratories?
01
Interpretive guidelines for laboratories are needed by:
02
- Laboratory managers and directors: They use the guidelines to establish a standardized framework for interpreting test results and ensuring consistency across different laboratory procedures and practices.
03
- Laboratory staff and technicians: The guidelines serve as a reference tool for understanding and interpreting test results accurately, especially when faced with complex or ambiguous cases.
04
- Accrediting bodies and regulatory agencies: These organizations may require laboratories to have interpretive guidelines in place to ensure quality assurance and adherence to industry standards.
05
- Healthcare professionals: Doctors, nurses, and other medical professionals rely on interpretive guidelines to interpret laboratory test results and make accurate diagnoses or treatment decisions.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I modify interpretive guidelines for laboratories without leaving Google Drive?
Simplify your document workflows and create fillable forms right in Google Drive by integrating pdfFiller with Google Docs. The integration will allow you to create, modify, and eSign documents, including interpretive guidelines for laboratories, without leaving Google Drive. Add pdfFiller’s functionalities to Google Drive and manage your paperwork more efficiently on any internet-connected device.
How do I edit interpretive guidelines for laboratories online?
With pdfFiller, it's easy to make changes. Open your interpretive guidelines for laboratories in the editor, which is very easy to use and understand. When you go there, you'll be able to black out and change text, write and erase, add images, draw lines, arrows, and more. You can also add sticky notes and text boxes.
How do I fill out interpretive guidelines for laboratories using my mobile device?
On your mobile device, use the pdfFiller mobile app to complete and sign interpretive guidelines for laboratories. Visit our website (https://edit-pdf-ios-android.pdffiller.com/) to discover more about our mobile applications, the features you'll have access to, and how to get started.
What is interpretive guidelines for laboratories?
Interpretive guidelines for laboratories provide clear instructions and standards that laboratories must follow to ensure compliance with regulations and to maintain the quality and accuracy of their testing processes.
Who is required to file interpretive guidelines for laboratories?
Laboratories that perform certain types of tests and are subject to regulatory oversight are required to file interpretive guidelines. This typically includes clinical laboratories and others that conduct regulated testing.
How to fill out interpretive guidelines for laboratories?
To fill out interpretive guidelines for laboratories, one must gather relevant information about the laboratory's operations, compliance measures, and specific testing procedures, and accurately complete any designated forms or documentation as required by the regulatory body.
What is the purpose of interpretive guidelines for laboratories?
The purpose of interpretive guidelines for laboratories is to ensure consistency in laboratory practices, enhance the quality of laboratory services, protect public health, and facilitate compliance with regulatory standards.
What information must be reported on interpretive guidelines for laboratories?
Information that must be reported typically includes laboratory certifications, test methodologies, quality control measures, compliance with safety standards, and any relevant performance data.
Fill out your interpretive guidelines for laboratories online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Interpretive Guidelines For Laboratories is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.

















