Get the free Technical Standards for Medical Laboratory Technician. Form that enrolling students ...
Show details
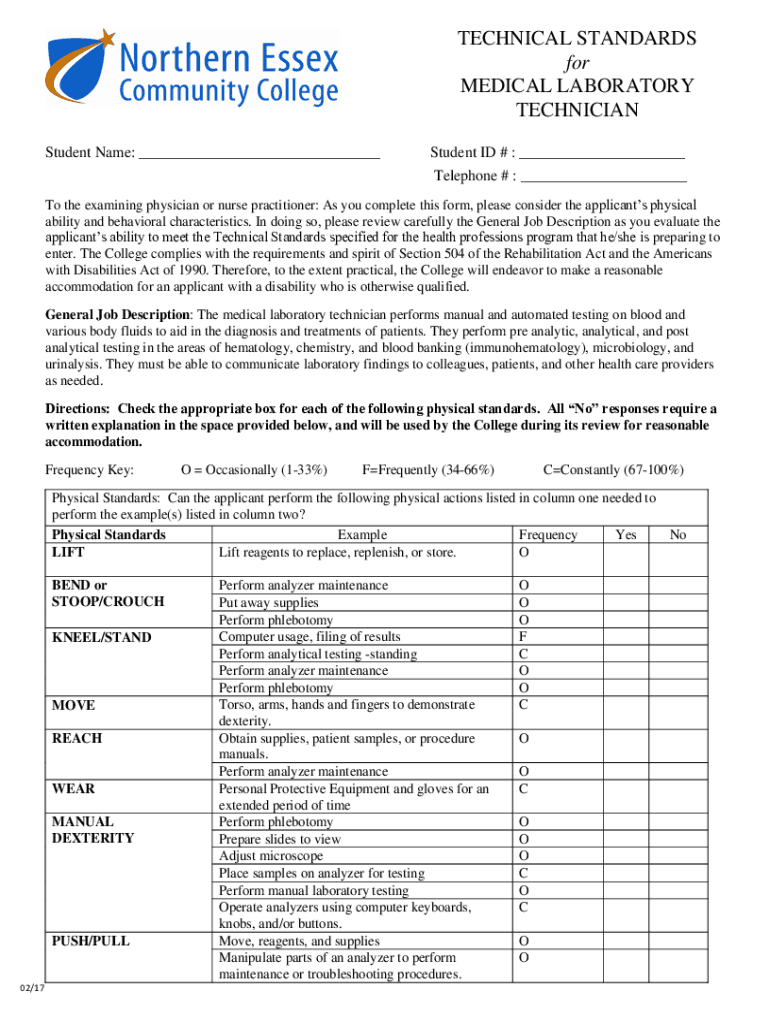
TECHNICAL STANDARDS for MEDICAL LABORATORY TECHNICIAN Student Name: Student ID # : Telephone # : To the examining physician or nurse practitioner: As you complete this form, please consider the applicants
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign technical standards for medical

Edit your technical standards for medical form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your technical standards for medical form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit technical standards for medical online
To use our professional PDF editor, follow these steps:
1
Log in to your account. Click on Start Free Trial and register a profile if you don't have one.
2
Upload a file. Select Add New on your Dashboard and upload a file from your device or import it from the cloud, online, or internal mail. Then click Edit.
3
Edit technical standards for medical. Add and change text, add new objects, move pages, add watermarks and page numbers, and more. Then click Done when you're done editing and go to the Documents tab to merge or split the file. If you want to lock or unlock the file, click the lock or unlock button.
4
Get your file. When you find your file in the docs list, click on its name and choose how you want to save it. To get the PDF, you can save it, send an email with it, or move it to the cloud.
pdfFiller makes dealing with documents a breeze. Create an account to find out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out technical standards for medical

How to fill out technical standards for medical
01
Start by understanding the specific technical standards required for medical purposes. These standards may include guidelines for equipment, protocols, software, data storage, and interoperability.
02
Familiarize yourself with the regulations and guidelines set forth by regulatory bodies such as the Food and Drug Administration (FDA) or the International Organization for Standardization (ISO). These organizations often provide detailed instructions on how to fill out technical standards.
03
Gather all the necessary information and documentation related to the medical device or system for which you are filling out the technical standards. This may include technical specifications, test reports, risk assessments, and validation documentation.
04
Follow the prescribed format for filling out the technical standards. This typically involves providing detailed information about the device or system, its intended use, performance requirements, safety features, and any associated risks.
05
Pay attention to any specific terminology or terminology conventions required in the technical standards. Use clear and concise language to describe the different aspects of the medical device or system.
06
Ensure that all the information provided is accurate, up-to-date, and supported by relevant evidence or documentation. Any claims or statements should be verifiable and backed by scientific or clinical data.
07
Review and proofread the filled-out technical standards multiple times to ensure accuracy and completeness. Seek feedback or assistance from experts or colleagues, if necessary.
08
Submit the filled-out technical standards according to the specified procedure or submission process. Keep a copy of the completed standards for future reference or audits.
09
Stay updated with any revisions or updates to the technical standards. Regularly review and update your own processes or systems to comply with the latest requirements.
10
Seek professional assistance or consultation if you have any doubts or difficulties in filling out the technical standards for medical purposes.
Who needs technical standards for medical?
01
Various stakeholders in the medical industry may need technical standards for medical purposes. This includes medical device manufacturers, healthcare providers, regulatory authorities, research institutions, and healthcare software developers.
02
Medical device manufacturers rely on technical standards to ensure their products comply with industry regulations and safety requirements. These standards provide guidelines and benchmarks for designing, manufacturing, and testing medical devices.
03
Healthcare providers, such as hospitals and clinics, may need technical standards to ensure interoperability, compatibility, and compliance with other devices and systems used in their facilities. These standards help in establishing seamless communication and integration among different medical technologies.
04
Regulatory authorities, like the FDA, require technical standards to assess the safety, effectiveness, and quality of medical devices. These standards assist in evaluating the performance, reliability, and risk management associated with medical devices.
05
Research institutions may use technical standards to conduct studies, experiments, or clinical trials involving medical devices. These standards help ensure consistency, comparability, and reproducibility of research outcomes.
06
Healthcare software developers rely on technical standards to create software solutions that meet industry requirements and standards. These standards help define data formats, interoperability, security measures, and regulatory compliance in healthcare software applications.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I make changes in technical standards for medical?
pdfFiller not only allows you to edit the content of your files but fully rearrange them by changing the number and sequence of pages. Upload your technical standards for medical to the editor and make any required adjustments in a couple of clicks. The editor enables you to blackout, type, and erase text in PDFs, add images, sticky notes and text boxes, and much more.
How do I fill out the technical standards for medical form on my smartphone?
You can quickly make and fill out legal forms with the help of the pdfFiller app on your phone. Complete and sign technical standards for medical and other documents on your mobile device using the application. If you want to learn more about how the PDF editor works, go to pdfFiller.com.
Can I edit technical standards for medical on an Android device?
With the pdfFiller Android app, you can edit, sign, and share technical standards for medical on your mobile device from any place. All you need is an internet connection to do this. Keep your documents in order from anywhere with the help of the app!
What is technical standards for medical?
Technical standards for medical refers to a set of guidelines and specifications that outline the expected quality, safety, and effectiveness of medical devices, equipment, and processes.
Who is required to file technical standards for medical?
Manufacturers, importers, and distributors of medical devices are typically required to file technical standards for medical to ensure compliance with regulatory requirements.
How to fill out technical standards for medical?
To fill out technical standards for medical, organizations must gather necessary documentation, ensure all specified requirements are met, complete the relevant forms accurately, and submit the forms to the appropriate regulatory body.
What is the purpose of technical standards for medical?
The purpose of technical standards for medical is to ensure that medical devices and procedures meet established safety and efficacy criteria, thus protecting public health.
What information must be reported on technical standards for medical?
Information that must be reported includes device specifications, manufacturing practices, safety and performance data, and compliance with relevant regulations and standards.
Fill out your technical standards for medical online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Technical Standards For Medical is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.

















