Get the free REACTIONS RATES AND REVERSIBLE REACTIONS- FORM 4 CHEMISTRY TOPICAL QUESTIONS. REACTI...
Show details
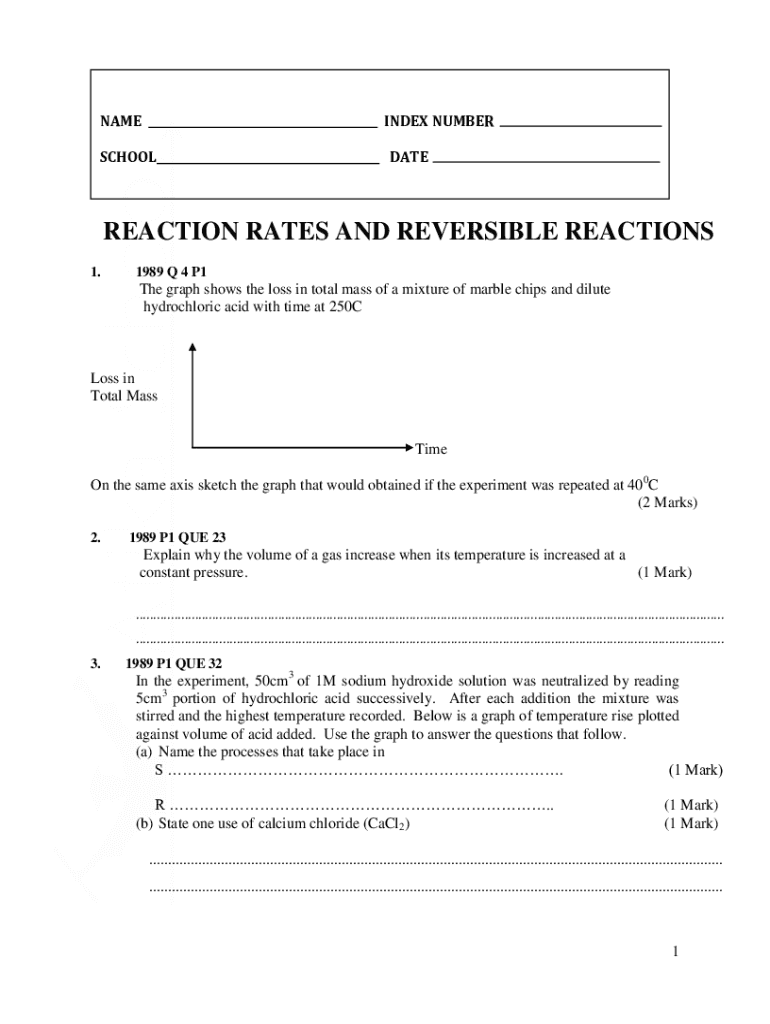
AMENDED NUMBERSCHOOLDATEREACTION RATES AND REVERSIBLE REACTIONS
1.1989 Q 4 P1The graph shows the loss in total mass of a mixture of marble chips and dilute
hydrochloric acid with time at 250CLoss
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign reactions rates and reversible

Edit your reactions rates and reversible form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your reactions rates and reversible form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit reactions rates and reversible online
Follow the guidelines below to benefit from a competent PDF editor:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit reactions rates and reversible. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Save your file. Select it in the list of your records. Then, move the cursor to the right toolbar and choose one of the available exporting methods: save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud.
With pdfFiller, dealing with documents is always straightforward.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out reactions rates and reversible

How to fill out reactions rates and reversible
01
To fill out reaction rates and reversible steps, follow these steps:
02
Identify all the reactants and products in the chemical reaction.
03
Write a balanced chemical equation for the reaction.
04
Determine the stoichiometry of the reaction, which includes the coefficients of the reactants and products.
05
Write down the rate expression for the reaction, which is the mathematical expression that describes how the rate of the reaction depends on the concentrations of the reactants.
06
Determine the rate constant for the reaction, which is a proportionality constant that relates the rate of the reaction to the concentration of the reactants.
07
Use experimental data to determine the values of the rate constant and the reaction order, if necessary.
08
Take into account any reversible steps in the reaction, which means considering the forward and reverse reactions and their respective rate constants.
09
Calculate the rate of the reaction using the rate expression, the rate constant, and the concentrations of the reactants.
10
Repeat steps 4-8 for any additional reactions or steps in the reaction mechanism.
11
Fill out the reaction rates and reversible steps in a table or graph, showing the rate of the reaction at different concentrations or conditions.
Who needs reactions rates and reversible?
01
Reaction rates and reversible steps are important for:
02
- Chemical engineers, who need to optimize reaction processes and design efficient reactors.
03
- Industrial chemists, who work on developing new chemical processes and improving existing ones in industries such as pharmaceuticals, petrochemicals, and materials.
04
- Environmental scientists, who study the kinetics of reactions in the atmosphere and in natural environments.
05
- Researchers in the field of biochemistry, who investigate enzyme kinetics and metabolic pathways.
06
- Students studying chemistry or chemical engineering, who need to understand and apply the principles of reaction rates and reversible steps in their coursework.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
Can I create an eSignature for the reactions rates and reversible in Gmail?
With pdfFiller's add-on, you may upload, type, or draw a signature in Gmail. You can eSign your reactions rates and reversible and other papers directly in your mailbox with pdfFiller. To preserve signed papers and your personal signatures, create an account.
How can I fill out reactions rates and reversible on an iOS device?
In order to fill out documents on your iOS device, install the pdfFiller app. Create an account or log in to an existing one if you have a subscription to the service. Once the registration process is complete, upload your reactions rates and reversible. You now can take advantage of pdfFiller's advanced functionalities: adding fillable fields and eSigning documents, and accessing them from any device, wherever you are.
Can I edit reactions rates and reversible on an Android device?
You can make any changes to PDF files, like reactions rates and reversible, with the help of the pdfFiller Android app. Edit, sign, and send documents right from your phone or tablet. You can use the app to make document management easier wherever you are.
What is reactions rates and reversible?
Reaction rates refer to the speed at which a chemical reaction occurs, while reversible reactions are processes that can proceed in both forward and backward directions, allowing the reactants to form products and vice versa.
Who is required to file reactions rates and reversible?
Entities engaged in chemical manufacturing or research that involve reactions rates and reversible processes are typically required to file related documentation.
How to fill out reactions rates and reversible?
To fill out forms related to reaction rates and reversible processes, follow the guidelines provided by the regulatory authority, ensuring all data regarding reaction kinetics, conditions, and reversibility is accurately included.
What is the purpose of reactions rates and reversible?
The purpose of studying reaction rates and reversible processes is to understand the dynamics of chemical reactions, optimize conditions for desired outcomes, and ensure compliance with safety and environmental regulations.
What information must be reported on reactions rates and reversible?
Required information includes the reaction conditions, rates of reaction, temperature, pressure, and any relevant data on the reversibility of the processes.
Fill out your reactions rates and reversible online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Reactions Rates And Reversible is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.


















