Get the free Frontiers Evaluating SARS-CoV-2 Seroconversion Following ...
Show details
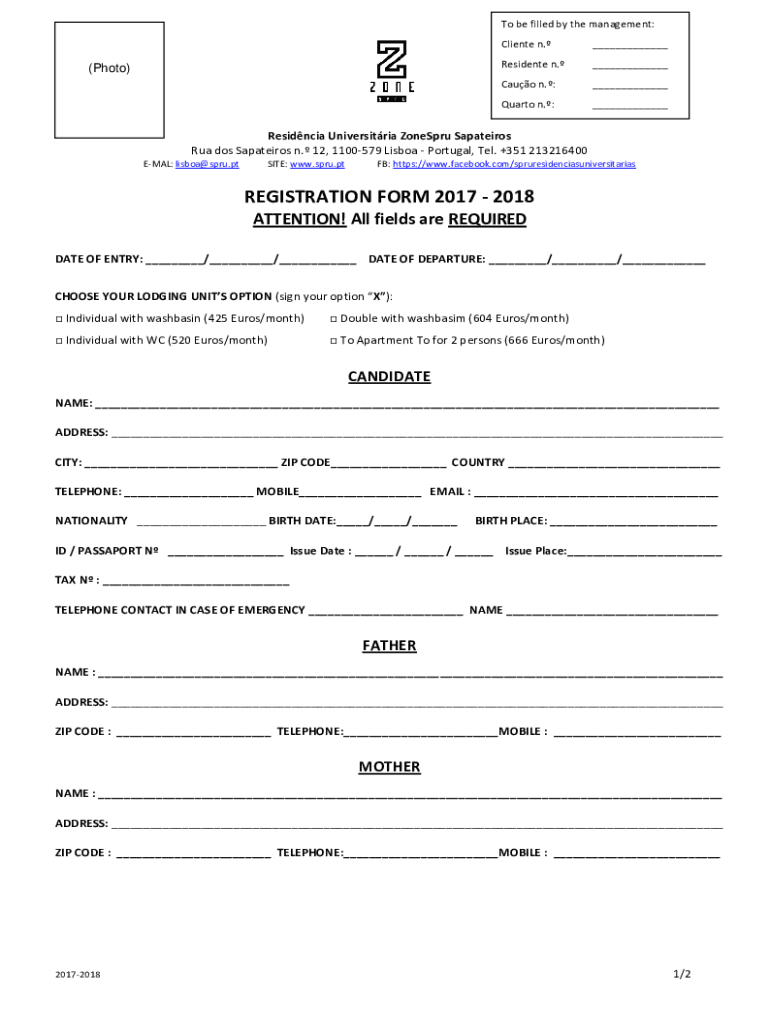
To be filled by the management:(Photo)Client n. Residents n. Cato n.: Quarto n.: Residence University Zones Spatial RUA dos Spatial n. 12, 1100579 Lisbon Portugal, Tel. +351 213216400 EMAIL: Lisbon
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign frontiers evaluating sars-cov-2 seroconversion

Edit your frontiers evaluating sars-cov-2 seroconversion form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your frontiers evaluating sars-cov-2 seroconversion form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit frontiers evaluating sars-cov-2 seroconversion online
Use the instructions below to start using our professional PDF editor:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit frontiers evaluating sars-cov-2 seroconversion. Rearrange and rotate pages, add new and changed texts, add new objects, and use other useful tools. When you're done, click Done. You can use the Documents tab to merge, split, lock, or unlock your files.
4
Save your file. Select it from your list of records. Then, move your cursor to the right toolbar and choose one of the exporting options. You can save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud, among other things.
It's easier to work with documents with pdfFiller than you can have believed. Sign up for a free account to view.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out frontiers evaluating sars-cov-2 seroconversion

How to fill out frontiers evaluating sars-cov-2 seroconversion
01
Collect blood samples from individuals who have potentially been exposed to SARS-CoV-2.
02
Use an ELISA or other immunoassay technique to detect the presence of antibodies against SARS-CoV-2 in the collected blood samples.
03
Follow the manufacturer's instructions for the specific assay kit being used.
04
Prepare the samples and reagents according to the assay protocol.
05
Incubate the samples with the assay reagents for the recommended amount of time.
06
Wash the samples to remove any unbound reagents.
07
Add the substrate solution and incubate for the designated time to allow the development of color.
08
Stop the reaction by adding a stop solution.
09
Measure the absorbance or fluorescence of each sample using a microplate reader.
10
Calculate the antibody concentrations or seroconversion status based on the obtained measurements.
11
Interpret the results according to the assay's cutoff values and established criteria.
12
Report the findings and provide appropriate recommendations for further actions or follow-up testing.
Who needs frontiers evaluating sars-cov-2 seroconversion?
01
Individuals who have been exposed to SARS-CoV-2 and want to confirm their seroconversion status.
02
Healthcare professionals involved in diagnosing and monitoring COVID-19 patients.
03
Researchers studying the immune response to SARS-CoV-2.
04
Public health agencies and officials tracking the spread of COVID-19 in a population.
05
Organizations planning to implement sero-surveys to assess the prevalence of SARS-CoV-2 antibodies in a community or certain population groups.
06
Individuals participating in vaccine trials to assess the development of protective antibodies.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I manage my frontiers evaluating sars-cov-2 seroconversion directly from Gmail?
frontiers evaluating sars-cov-2 seroconversion and other documents can be changed, filled out, and signed right in your Gmail inbox. You can use pdfFiller's add-on to do this, as well as other things. When you go to Google Workspace, you can find pdfFiller for Gmail. You should use the time you spend dealing with your documents and eSignatures for more important things, like going to the gym or going to the dentist.
How do I make edits in frontiers evaluating sars-cov-2 seroconversion without leaving Chrome?
Install the pdfFiller Google Chrome Extension in your web browser to begin editing frontiers evaluating sars-cov-2 seroconversion and other documents right from a Google search page. When you examine your documents in Chrome, you may make changes to them. With pdfFiller, you can create fillable documents and update existing PDFs from any internet-connected device.
Can I create an electronic signature for signing my frontiers evaluating sars-cov-2 seroconversion in Gmail?
Upload, type, or draw a signature in Gmail with the help of pdfFiller’s add-on. pdfFiller enables you to eSign your frontiers evaluating sars-cov-2 seroconversion and other documents right in your inbox. Register your account in order to save signed documents and your personal signatures.
What is frontiers evaluating sars-cov-2 seroconversion?
Frontiers evaluating SARS-CoV-2 seroconversion is a research initiative aimed at assessing the immune response and antibody development in individuals who have been exposed to the virus. It involves studying the presence of antibodies in the blood to determine the serological status of a population.
Who is required to file frontiers evaluating sars-cov-2 seroconversion?
Individuals or organizations conducting research or studies related to SARS-CoV-2 seroconversion are typically required to file the relevant data. This may include healthcare professionals, researchers, or institutions involved in epidemiological surveys.
How to fill out frontiers evaluating sars-cov-2 seroconversion?
To fill out the frontiers evaluating SARS-CoV-2 seroconversion, you need to collect patient or participant data, perform serological tests, and input the results along with demographic information into the designated reporting forms or systems set up by the governing body overseeing the evaluation.
What is the purpose of frontiers evaluating sars-cov-2 seroconversion?
The purpose is to understand the extent of past infections within a population, gauge community immunity levels, and inform public health strategies for controlling the spread of COVID-19.
What information must be reported on frontiers evaluating sars-cov-2 seroconversion?
Information that must be reported includes participant demographics, serological test results, timing of sample collection, and possible exposure history to SARS-CoV-2.
Fill out your frontiers evaluating sars-cov-2 seroconversion online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Frontiers Evaluating Sars-Cov-2 Seroconversion is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.



















