FDA 3537 2020-2025 free printable template
Show details
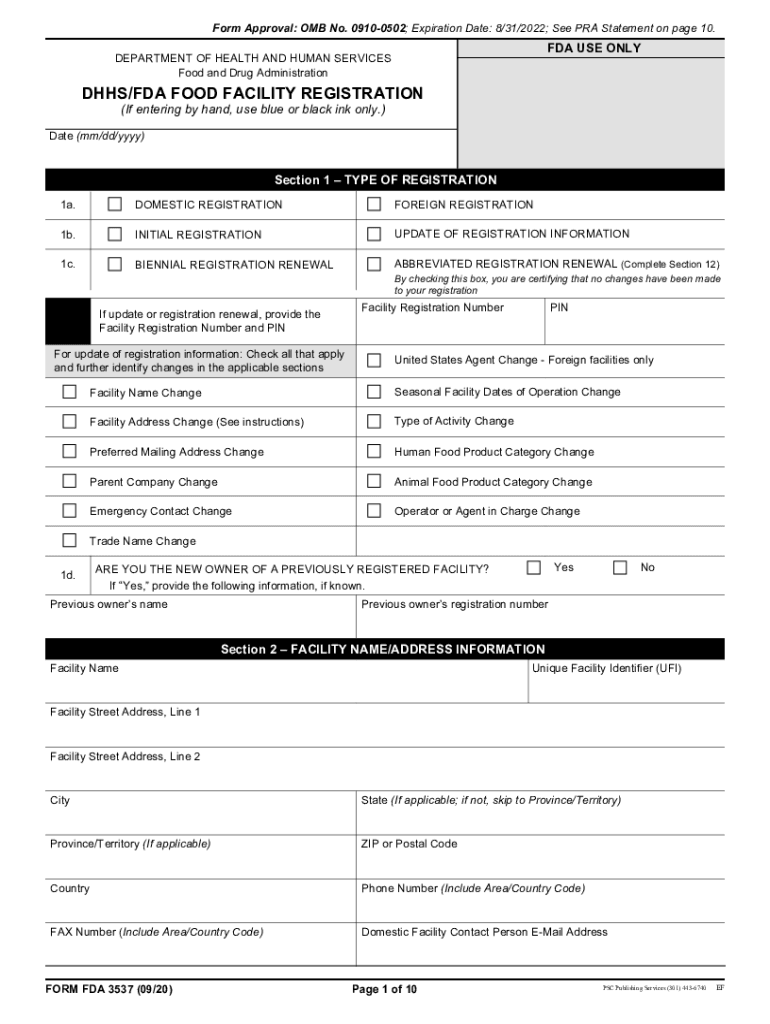
Form Approval OMB No. 0910-0502 Expiration Date 03/31/2013 See PRA Statement on page 10. FDA USE ONLY DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration DHHS/FDA FOOD FACILITY REGISTRATION If entering by hand use blue or black ink only. Section 11 OWNER OPERATOR OR AGENT-IN-CHARGE INFORMATION Name of Entity or Individual Who Is the Owner Operator or Agent-in-Charge Provide the following information if different from all other sections on the form. If the information is the...
pdfFiller is not affiliated with any government organization
Get, Create, Make and Sign form fda 3537

Edit your form food facility form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your fda form 3537 form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing fda food facility registration form online
In order to make advantage of the professional PDF editor, follow these steps below:
1
Log into your account. If you don't have a profile yet, click Start Free Trial and sign up for one.
2
Upload a file. Select Add New on your Dashboard and upload a file from your device or import it from the cloud, online, or internal mail. Then click Edit.
3
Edit form food facility registration. Replace text, adding objects, rearranging pages, and more. Then select the Documents tab to combine, divide, lock or unlock the file.
4
Save your file. Select it from your list of records. Then, move your cursor to the right toolbar and choose one of the exporting options. You can save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud, among other things.
pdfFiller makes working with documents easier than you could ever imagine. Register for an account and see for yourself!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
FDA 3537 Form Versions
Version
Form Popularity
Fillable & printabley
How to fill out 3537 food registration form

How to fill out FDA 3537
01
Begin by obtaining a copy of FDA Form 3537, which can be downloaded from the FDA website.
02
Fill out the applicant's name and address in the designated fields.
03
Provide the facility name and address where the agent will be located.
04
Indicate the type of submission (e.g., new submission, amendment).
05
Specify the reason for the submission, such as a request for an establishment registration.
06
Ensure all required signatures are obtained from the responsible parties.
07
Review the form for any errors or missing information before submission.
08
Submit the completed form via email or through the appropriate FDA submission portal.
Who needs FDA 3537?
01
FDA Form 3537 is needed by foreign entities that appoint a U.S. agent for their FDA-regulated products.
02
It is required for businesses and manufacturers seeking to comply with FDA regulations on drug or device imports.
Fill
fda form 3537 online download
: Try Risk Free






People Also Ask about form fda 3537 online
How long does an FDA food facility registration take?
How long does FDA Registration take? FDA Specialist can usually complete the registration for a company within 2-3 business days if provided all the necessary information and the company completes all required steps. The timeframes for certain products may also depend on how quickly FDA can process the applications.
How do I find FDA food facility registration?
Log into the FDA Industry Systems (FIS). Choose "FURLS Food Facility Registration Module (FFRM)" from the list of available systems on Account Management Home Page. This guide includes instructions on: Section 1 – Type of Registration.
Who needs FDA food facility registration?
Food. Owners, operators, or agents in charge of domestic or foreign facilities that manufacture/process, pack, or hold food for consumption in the U.S. are required to register the facility with the FDA.
How long does it take to get FDA approval?
The FDA approval process can take between one week and eight months, depending on whether you self-register, submit a 510(k) application or submit a Premarket Approval (PMA) application. Bringing a medical device to market is not a fast process.
What is food facility registration?
U.S. FDA Food Facility Registration. Facilities that manufacture, process, pack, or store food, beverages, or dietary supplements that may be consumed in the United States must register with the U.S. Food and Drug Administration (FDA).
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I edit 3537 facility blank from Google Drive?
Using pdfFiller with Google Docs allows you to create, amend, and sign documents straight from your Google Drive. The add-on turns your 3537 facility pdf into a dynamic fillable form that you can manage and eSign from anywhere.
How do I execute 3537 registration printable online?
Filling out and eSigning fda form 3537 online test create is now simple. The solution allows you to change and reorganize PDF text, add fillable fields, and eSign the document. Start a free trial of pdfFiller, the best document editing solution.
How can I fill out fda form 3537 online on an iOS device?
Download and install the pdfFiller iOS app. Then, launch the app and log in or create an account to have access to all of the editing tools of the solution. Upload your dr 1 form 2020-2025 from your device or cloud storage to open it, or input the document URL. After filling out all of the essential areas in the document and eSigning it (if necessary), you may save it or share it with others.
What is FDA 3537?
FDA 3537 is a form used by the U.S. Food and Drug Administration for the reporting of adverse events related to the use of medical devices.
Who is required to file FDA 3537?
Manufacturers, importers, and device user facilities are required to file FDA 3537 when they become aware of adverse events related to medical devices.
How to fill out FDA 3537?
To fill out FDA 3537, you need to provide information regarding the device, the adverse event, patient details, and reporter details, adhering to the specific guidelines set by the FDA.
What is the purpose of FDA 3537?
The purpose of FDA 3537 is to collect information about adverse events to enhance the safety and effectiveness of medical devices in the market.
What information must be reported on FDA 3537?
FDA 3537 must include details like the device identification, patient age and gender, date of event, description of the adverse event, and any relevant medical history.
Fill out your dr 1 form 2020-2025 online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Dr 1 Form 2020-2025 is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.























