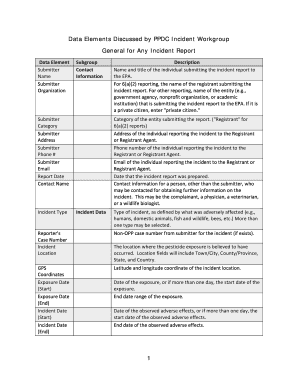
Get the free FDA MedWatch Form 3500 (Voluntary Reporting). Misc Forms
Show details
U.S. Department of Health and Human ServicesMEDWATCHFor VOLUNTARY reporting of adverse events, product problems and product use errors FDA Safety Information and Adverse Event Reporting Program Page
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign fda medwatch form 3500

Edit your fda medwatch form 3500 form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your fda medwatch form 3500 form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit fda medwatch form 3500 online
Follow the guidelines below to take advantage of the professional PDF editor:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Upload a file. Select Add New on your Dashboard and upload a file from your device or import it from the cloud, online, or internal mail. Then click Edit.
3
Edit fda medwatch form 3500. Add and replace text, insert new objects, rearrange pages, add watermarks and page numbers, and more. Click Done when you are finished editing and go to the Documents tab to merge, split, lock or unlock the file.
4
Save your file. Select it from your records list. Then, click the right toolbar and select one of the various exporting options: save in numerous formats, download as PDF, email, or cloud.
With pdfFiller, it's always easy to work with documents. Try it out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out fda medwatch form 3500

How to fill out fda medwatch form 3500
01
To fill out FDA MedWatch Form 3500, follow these steps:
02
Download the form from the FDA MedWatch website or obtain a physical copy from a healthcare provider.
03
Begin by providing your personal information, including your name, address, phone number, and email address.
04
Indicate the type of reporter you are by selecting the appropriate category (e.g., healthcare professional, consumer).
05
Specify the patient information, such as their age, gender, weight, and relevant medical history.
06
Describe the adverse event or product problem in detail, including when it occurred, how it was discovered, and any actions taken.
07
Provide information about the suspect medication or product, including the name, strength, dosage form, and manufacturer.
08
Include any additional attachments, such as lab reports or photos, if necessary.
09
Sign and date the form to acknowledge the accuracy of the information provided.
10
Submit the completed form to the FDA using the appropriate submission method as mentioned on the form or the FDA MedWatch website.
11
It is recommended to review the FDA MedWatch instructions for detailed guidance on filling out form 3500.
Who needs fda medwatch form 3500?
01
FDA MedWatch Form 3500 is needed by anyone, including healthcare professionals and consumers, who wants to report adverse events, product problems, or errors related to FDA-regulated products.
02
This form allows individuals to communicate important safety information to the FDA, enabling them to track potential issues, identify trends, and take appropriate regulatory actions to ensure public health and safety.
03
Healthcare professionals, patients, caregivers, and other concerned individuals can use this form to raise awareness about any potential risks, adverse reactions, or quality problems associated with FDA-regulated products.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I send fda medwatch form 3500 to be eSigned by others?
To distribute your fda medwatch form 3500, simply send it to others and receive the eSigned document back instantly. Post or email a PDF that you've notarized online. Doing so requires never leaving your account.
Can I create an electronic signature for the fda medwatch form 3500 in Chrome?
As a PDF editor and form builder, pdfFiller has a lot of features. It also has a powerful e-signature tool that you can add to your Chrome browser. With our extension, you can type, draw, or take a picture of your signature with your webcam to make your legally-binding eSignature. Choose how you want to sign your fda medwatch form 3500 and you'll be done in minutes.
Can I edit fda medwatch form 3500 on an iOS device?
You can. Using the pdfFiller iOS app, you can edit, distribute, and sign fda medwatch form 3500. Install it in seconds at the Apple Store. The app is free, but you must register to buy a subscription or start a free trial.
What is fda medwatch form 3500?
FDA MedWatch Form 3500 is a form used for reporting adverse events, product problems, and medication errors to the FDA.
Who is required to file fda medwatch form 3500?
Healthcare professionals, patients, and manufacturers are required to file FDA MedWatch Form 3500.
How to fill out fda medwatch form 3500?
FDA MedWatch Form 3500 can be filled out online on the FDA's website or by submitting a paper form via mail or fax.
What is the purpose of fda medwatch form 3500?
The purpose of FDA MedWatch Form 3500 is to collect and report information on adverse events, product problems, and medication errors related to FDA-regulated products.
What information must be reported on fda medwatch form 3500?
Information such as details of the adverse event, product information, patient information, and contact information must be reported on FDA MedWatch Form 3500.
Fill out your fda medwatch form 3500 online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Fda Medwatch Form 3500 is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.