Get the free Clinical Research Study First Point of Contact Screening
Show details
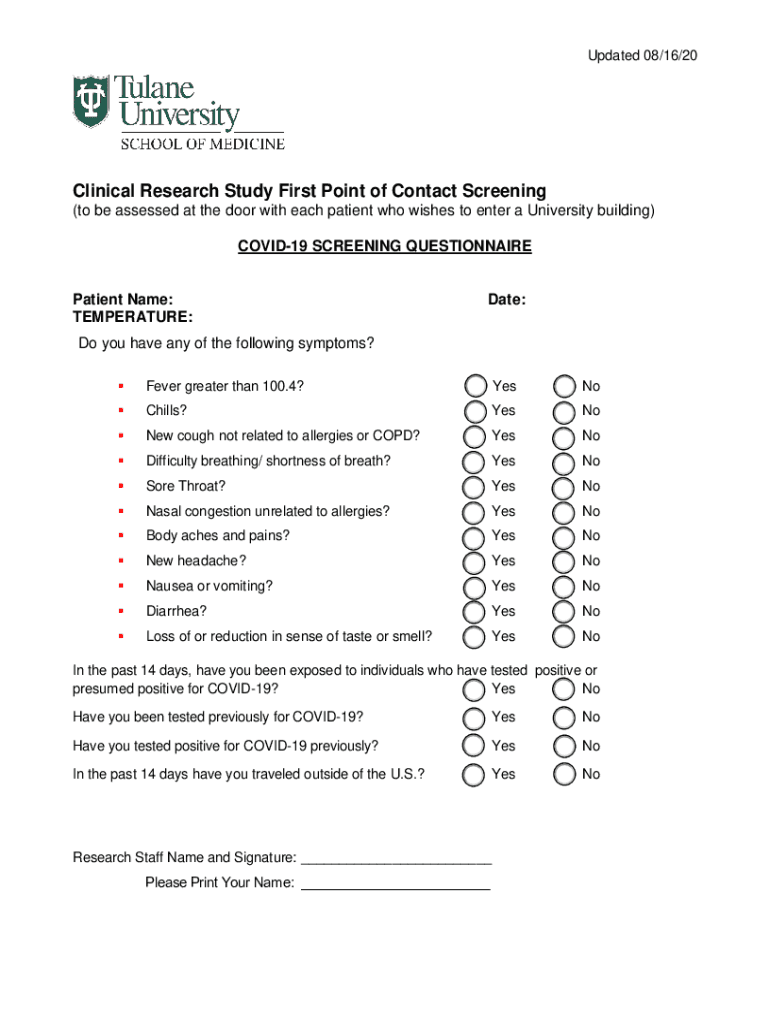
Updated 08/16/20Clinical Research Study First Point of Contact Screening
(to be assessed at the door with each patient who wishes to enter a University building)
COVID-19 SCREENING QUESTIONNAIREPatient
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign clinical research study first

Edit your clinical research study first form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your clinical research study first form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing clinical research study first online
Use the instructions below to start using our professional PDF editor:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Upload a document. Select Add New on your Dashboard and transfer a file into the system in one of the following ways: by uploading it from your device or importing from the cloud, web, or internal mail. Then, click Start editing.
3
Edit clinical research study first. Rearrange and rotate pages, add new and changed texts, add new objects, and use other useful tools. When you're done, click Done. You can use the Documents tab to merge, split, lock, or unlock your files.
4
Get your file. Select the name of your file in the docs list and choose your preferred exporting method. You can download it as a PDF, save it in another format, send it by email, or transfer it to the cloud.
pdfFiller makes working with documents easier than you could ever imagine. Create an account to find out for yourself how it works!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out clinical research study first

How to fill out clinical research study first
01
To fill out a clinical research study form, follow these steps:
02
Read the study requirements carefully to ensure you meet the eligibility criteria.
03
Gather all necessary documents such as medical records, identification, and any previous test results.
04
Contact the research team or the study coordinator to express your interest and ask for any additional information or forms.
05
Schedule an appointment with the research site or clinic.
06
Arrive at the appointed time and provide all required information and documentation.
07
Fill out the study form accurately and honestly, answering all questions to the best of your ability.
08
Ask any questions you may have about the study or the form before submitting it.
09
Review the completed form for any errors or missing information.
10
Submit the form to the research team or the study coordinator.
11
Follow any further instructions provided by the research team or the study coordinator, if applicable.
Who needs clinical research study first?
01
Clinical research studies are typically needed by individuals who meet specific criteria outlined by the study protocol and have a desire to contribute to medical research.
02
The following individuals may need to participate in a clinical research study first:
03
- Patients diagnosed with a particular medical condition targeted by the study.
04
- Individuals who have exhausted standard treatment options and are seeking alternative or experimental interventions.
05
- Healthy volunteers willing to assist in the advancement of medical knowledge.
06
- Individuals who have a family history of a certain disease or condition being studied.
07
- Those who wish to access potentially life-saving treatments not yet approved for general use.
08
- Individuals seeking a deeper understanding of their own medical conditions.
09
It is important to note that eligibility criteria for clinical research studies can vary greatly depending on the nature and purpose of the study.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I modify clinical research study first without leaving Google Drive?
By combining pdfFiller with Google Docs, you can generate fillable forms directly in Google Drive. No need to leave Google Drive to make edits or sign documents, including clinical research study first. Use pdfFiller's features in Google Drive to handle documents on any internet-connected device.
How do I execute clinical research study first online?
pdfFiller has made it simple to fill out and eSign clinical research study first. The application has capabilities that allow you to modify and rearrange PDF content, add fillable fields, and eSign the document. Begin a free trial to discover all of the features of pdfFiller, the best document editing solution.
How do I edit clinical research study first online?
pdfFiller not only allows you to edit the content of your files but fully rearrange them by changing the number and sequence of pages. Upload your clinical research study first to the editor and make any required adjustments in a couple of clicks. The editor enables you to blackout, type, and erase text in PDFs, add images, sticky notes and text boxes, and much more.
What is clinical research study first?
Clinical research study first refers to the initial phase of a study focusing on evaluating the safety and efficacy of a new treatment or intervention.
Who is required to file clinical research study first?
The principal investigator or research team is typically responsible for filing the clinical research study first.
How to fill out clinical research study first?
The clinical research study first can be filled out following the specific guidelines provided by the regulatory authorities or Institutional Review Board overseeing the study.
What is the purpose of clinical research study first?
The purpose of clinical research study first is to gather preliminary data on the safety and potential effectiveness of a new intervention before moving on to larger studies.
What information must be reported on clinical research study first?
The clinical research study first report should include details on the study design, methodology, participants, outcomes, and any adverse events observed during the study.
Fill out your clinical research study first online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Clinical Research Study First is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.


















