UK Kings Health Partners Clinical Trials Office Serious Adverse Event Form 2020-2026 free printable template
Show details
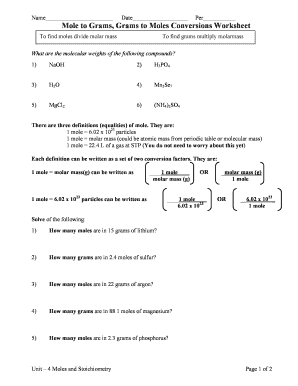
Serious Adverse Event Form Kings Health Partners Clinical Trials OfficeMax to: jcto.pharmacovigilance@kcl.ac.ukA partnership for clinical researchEudraCT Number:Participant Gender:Participant Randomization
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign UK Kings Health Partners Clinical Trials

Edit your UK Kings Health Partners Clinical Trials form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your UK Kings Health Partners Clinical Trials form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit UK Kings Health Partners Clinical Trials online
Use the instructions below to start using our professional PDF editor:
1
Log in. Click Start Free Trial and create a profile if necessary.
2
Upload a document. Select Add New on your Dashboard and transfer a file into the system in one of the following ways: by uploading it from your device or importing from the cloud, web, or internal mail. Then, click Start editing.
3
Edit UK Kings Health Partners Clinical Trials. Rearrange and rotate pages, insert new and alter existing texts, add new objects, and take advantage of other helpful tools. Click Done to apply changes and return to your Dashboard. Go to the Documents tab to access merging, splitting, locking, or unlocking functions.
4
Get your file. When you find your file in the docs list, click on its name and choose how you want to save it. To get the PDF, you can save it, send an email with it, or move it to the cloud.
With pdfFiller, it's always easy to work with documents.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
UK Kings Health Partners Clinical Trials Office Serious Adverse Event Form Form Versions
Version
Form Popularity
Fillable & printabley
4.9 Satisfied (32 Votes)
4.7 Satisfied (47 Votes)
How to fill out UK Kings Health Partners Clinical Trials

How to fill out UK Kings Health Partners Clinical Trials Office
01
Visit the UK Kings Health Partners website to access the Clinical Trials Office section.
02
Locate the application form for clinical trial submissions.
03
Fill in the required sections of the form, including trial title, description, and objectives.
04
Provide details about the research team, including names, roles, and contact information.
05
Include information on trial funding and sponsorship.
06
Outline participant eligibility criteria and recruitment strategies.
07
Submit any required ethical approvals or supporting documents.
08
Review all sections for accuracy and completeness before final submission.
09
Await confirmation of receipt from the Clinical Trials Office.
Who needs UK Kings Health Partners Clinical Trials Office?
01
Researchers looking to conduct clinical trials in the UK.
02
Healthcare professionals involved in clinical research.
03
Pharmaceutical companies seeking to register trials.
04
Participants interested in taking part in clinical trials.
05
Regulatory bodies needing oversight of clinical studies.
Fill
form
: Try Risk Free






People Also Ask about
What is adverse event reporting requirement?
An investigator (if he/she is not a sponsor-investigator) must report to the sponsor any serious adverse event within 24 hours of investigator learning about the event, whether or not considered drug related, including those listed in the protocol or investigator brochure and must include an assessment of whether there
What are the 4 criteria for adverse event reporting?
The minimum dataset required to consider information as a reportable AE is indeed minimal, namely (1) an identifiable patient, (2) an identifiable reporter, (3) product exposure, and (4) an event.
How do you write a adverse event report?
How to write an serious adverse event narrative? Patient details. Study details. Patient history (medical history, concomitant diseases, family history, and concomitant drugs) Details of the study drug. Event description and treatment details. Laboratory tests information. Action taken with the study drug. Outcome of event/s.
What are the FDA requirements for reporting adverse events?
Unexpected serious suspected adverse reactions and observations from animal studies suggesting significant risk to human subjects must be reported to FDA as soon as possible but no later than within 15 calendar days following the sponsor's initial receipt of the information.
What is an adverse event summary example?
An adverse event (AE) may be: A physical event; for example, rash. A psychological event; for example, altered cognition. A laboratory event; for example, elevated creatinine.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I fill out the UK Kings Health Partners Clinical Trials form on my smartphone?
On your mobile device, use the pdfFiller mobile app to complete and sign UK Kings Health Partners Clinical Trials. Visit our website (https://edit-pdf-ios-android.pdffiller.com/) to discover more about our mobile applications, the features you'll have access to, and how to get started.
How can I fill out UK Kings Health Partners Clinical Trials on an iOS device?
Make sure you get and install the pdfFiller iOS app. Next, open the app and log in or set up an account to use all of the solution's editing tools. If you want to open your UK Kings Health Partners Clinical Trials, you can upload it from your device or cloud storage, or you can type the document's URL into the box on the right. After you fill in all of the required fields in the document and eSign it, if that is required, you can save or share it with other people.
Can I edit UK Kings Health Partners Clinical Trials on an Android device?
You can edit, sign, and distribute UK Kings Health Partners Clinical Trials on your mobile device from anywhere using the pdfFiller mobile app for Android; all you need is an internet connection. Download the app and begin streamlining your document workflow from anywhere.
What is UK Kings Health Partners Clinical Trials Office?
UK Kings Health Partners Clinical Trials Office is a facility that supports the planning, management, and conduct of clinical trials in a collaborative environment, ensuring compliance with regulatory requirements and promoting best practices in clinical research.
Who is required to file UK Kings Health Partners Clinical Trials Office?
Researchers, sponsors, and institutions involved in conducting clinical trials within the UK Kings Health Partners network are required to file with the Clinical Trials Office.
How to fill out UK Kings Health Partners Clinical Trials Office?
To fill out the UK Kings Health Partners Clinical Trials Office application, follow the designated process outlined on their official website, including gathering necessary documentation, completing forms accurately, and submitting them as per the submission guidelines.
What is the purpose of UK Kings Health Partners Clinical Trials Office?
The purpose of the UK Kings Health Partners Clinical Trials Office is to facilitate efficient conduct of clinical research, provide support and guidance to researchers, ensure compliance with ethical standards, and ultimately enhance patient care through advancements in medical science.
What information must be reported on UK Kings Health Partners Clinical Trials Office?
Information that must be reported includes the study protocol, informed consent documents, regulatory approvals, participant recruitment strategies, safety monitoring plans, and any amendments to the protocol throughout the trial.
Fill out your UK Kings Health Partners Clinical Trials online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

UK Kings Health Partners Clinical Trials is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.