Get the free IMMUNE CHECKPOINT INHIBITORS: CLINICAL TRIAL TRACKER
Show details
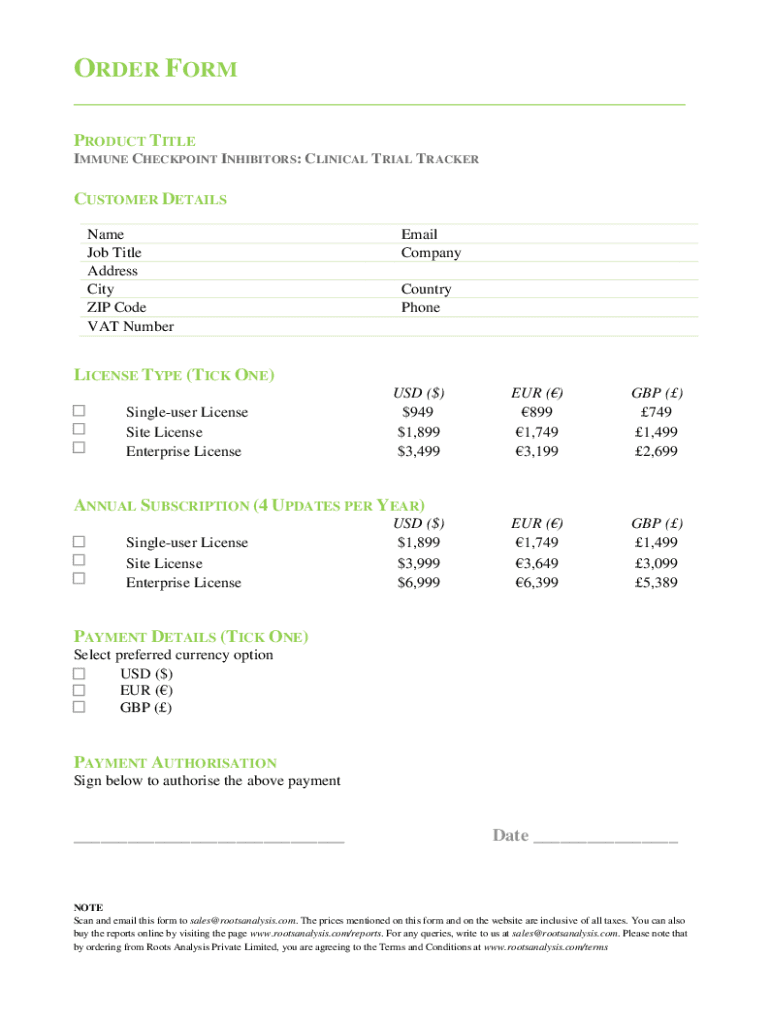
ORDER FORM
PRODUCT TITLE
IMMUNE CHECKPOINT INHIBITORS: CLINICAL TRIAL TRACKERCUSTOMER DETAILS
Name
Job Title
Address
City
ZIP Code
VAT NumberEmail
Company
Country
PhoneLICENSE TYPE (TICK ONE)
Single
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign immune checkpoint inhibitors clinical

Edit your immune checkpoint inhibitors clinical form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your immune checkpoint inhibitors clinical form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing immune checkpoint inhibitors clinical online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Log in. Click Start Free Trial and create a profile if necessary.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit immune checkpoint inhibitors clinical. Replace text, adding objects, rearranging pages, and more. Then select the Documents tab to combine, divide, lock or unlock the file.
4
Save your file. Select it from your list of records. Then, move your cursor to the right toolbar and choose one of the exporting options. You can save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud, among other things.
With pdfFiller, it's always easy to work with documents. Try it out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out immune checkpoint inhibitors clinical

How to fill out immune checkpoint inhibitors clinical
01
Identify the specific immune checkpoint inhibitor clinical trial that you are interested in.
02
Gather all relevant patient information and medical records.
03
Ensure that the patient meets the eligibility criteria for the specific clinical trial.
04
Consult with the patient's primary physician or oncologist to discuss the potential benefits and risks of participating in the clinical trial.
05
Obtain informed consent from the patient or their legal representative.
06
Complete all required documentation and paperwork for enrollment in the clinical trial.
07
Follow the specific instructions provided by the clinical trial coordinator for filling out the necessary forms and questionnaires.
08
Submit the completed documentation and forms to the designated clinical trial site or research institution.
09
Wait for confirmation of enrollment and further instructions from the clinical trial coordinator.
10
Comply with all follow-up visits and procedures as outlined in the clinical trial protocol.
Who needs immune checkpoint inhibitors clinical?
01
Patients with certain types of cancer, such as melanoma, lung cancer, bladder cancer, or kidney cancer, may benefit from immune checkpoint inhibitors clinical trials.
02
Patients who have not responded well to conventional treatments, such as chemotherapy or radiation therapy, may be considered for immune checkpoint inhibitors clinical trials.
03
Patients who are willing to participate in cutting-edge research and potentially receive innovative treatments for their cancer may be candidates for immune checkpoint inhibitors clinical trials.
04
However, each clinical trial has specific eligibility criteria, so it is important to consult with a healthcare professional or the clinical trial coordinator to determine if a patient is a suitable candidate for immune checkpoint inhibitors clinical trials.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I edit immune checkpoint inhibitors clinical from Google Drive?
Simplify your document workflows and create fillable forms right in Google Drive by integrating pdfFiller with Google Docs. The integration will allow you to create, modify, and eSign documents, including immune checkpoint inhibitors clinical, without leaving Google Drive. Add pdfFiller’s functionalities to Google Drive and manage your paperwork more efficiently on any internet-connected device.
How do I make changes in immune checkpoint inhibitors clinical?
pdfFiller allows you to edit not only the content of your files, but also the quantity and sequence of the pages. Upload your immune checkpoint inhibitors clinical to the editor and make adjustments in a matter of seconds. Text in PDFs may be blacked out, typed in, and erased using the editor. You may also include photos, sticky notes, and text boxes, among other things.
How do I edit immune checkpoint inhibitors clinical in Chrome?
Install the pdfFiller Google Chrome Extension in your web browser to begin editing immune checkpoint inhibitors clinical and other documents right from a Google search page. When you examine your documents in Chrome, you may make changes to them. With pdfFiller, you can create fillable documents and update existing PDFs from any internet-connected device.
What is immune checkpoint inhibitors clinical?
Immune checkpoint inhibitors clinical refers to a type of cancer therapy that blocks proteins that inhibit the immune response, allowing the immune system to better recognize and attack cancer cells.
Who is required to file immune checkpoint inhibitors clinical?
Researchers, clinical trial sponsors, and healthcare institutions conducting studies on immune checkpoint inhibitors are typically required to file clinical data.
How to fill out immune checkpoint inhibitors clinical?
To fill out immune checkpoint inhibitors clinical, one must follow the specific guidelines set by regulatory agencies, providing detailed information on the study design, patient demographics, treatment protocols, and outcomes.
What is the purpose of immune checkpoint inhibitors clinical?
The purpose of immune checkpoint inhibitors clinical is to evaluate the efficacy and safety of these therapies in treating cancer, as well as to understand their mechanisms of action.
What information must be reported on immune checkpoint inhibitors clinical?
Information that must be reported includes patient enrollment data, treatment protocols, efficacy results, adverse events, and any relevant demographic or clinical characteristics.
Fill out your immune checkpoint inhibitors clinical online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Immune Checkpoint Inhibitors Clinical is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.




















