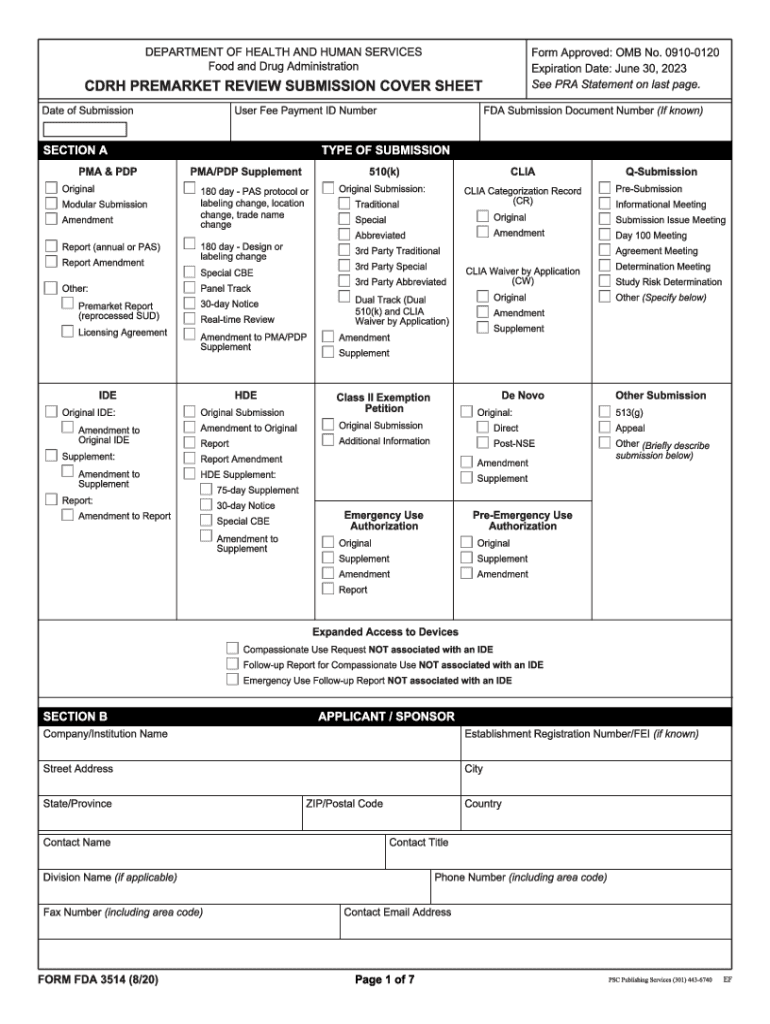
FDA 3514 2020-2025 free printable template
Get, Create, Make and Sign fda form 3514



Editing fda 3514 online
Uncompromising security for your PDF editing and eSignature needs
FDA 3514 Form Versions
How to fill out fda 3514 form pdf

How to fill out FDA 3514
Who needs FDA 3514?
Video instructions and help with filling out and completing cdrh premarket review submission cover sheet
Instructions and Help about fda sheet form
Hello everyone and welcome to registrar Corpse webinar entitled filing prior notice with FDA my name is Jonathan Rhodes a marketing specialist at registrar Corp and today's moderator the presentation will conclude with a live questions and answer session if we run out of time we are also happy to respond to your questions by email you may submit a written question any time during the webinar by using the ask a question feature in the top center of your webinar screen a recorded copy of this presentation will be sent to all registrants I'd like to introduce our speaker today crystal hunter holds a Master of Science degree in acquisition and supply chain management as well as a Master of Business Administration MS Hunter has experience in contract management corporate sales and business management MS Hunter began her career with registrar Corp in 2016 in the client services department she assists new and current clients with a variety of compliance in the food and beverage medical device drugs and cosmetics industries in order to market their products into the United States I'd like to go ahead and begin crystal Thank You, Jonathan hello everyone, and thank you for attending lets get started lets first discuss what prior notice is the US Food and Drug Administration requires the filing of prior notice for all food beverage and dietary supplement shipments entering the United States for both humans and animals by filing prior notice you are providing to the FDA important information of incoming food shipments to the US helping them to ensure the food safety for humans and animals upon receipt of prior notice FDA will issue a confirmation number in the form of a barcode pictured here that must accompany most food shipments this is not to be considered FDA approval this is a notification to the FDA only FDA still has the right to review and hold the shipment for further clarification if they feel this is necessary now let's discuss who can file prior notice anyone with knowledge of the shipment may file the prior notice to include the exporter importer third party such as a logistics company and manufacturers depending on the mode of transportation will affect how early you are required to submit your prior notice if your shipment is coming by Road you should submit your prior notice two hours before arrival by rail four hours prior to arrival by air four hours prior to arrival by water eight hours prior to arrival and if you are shipping your package by international mail you should file your prior notice before placing your package in the postal system lastly please remember that you should file your prior notice no more than 15 calendar days before the shipment is due to arrive now you may ask what information must be included in my prior notice there are numerous pieces of required information that must be submitted with each prior notice for instance information about the manufacturer submitter transmitter shipper an importer owner or ultimate...






People Also Ask about fda cover sheet form
Is FDA form 3514 required?
What is FDA form 3514?
What are the requirements for 510k reporting?
What is FDA Form 3514?
What is form 3454?
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I make changes in fda 3514 sheet form?
Can I create an eSignature for the premarket cover sheet fill in Gmail?
How do I edit fda cdrh form straight from my smartphone?
What is FDA 3514?
Who is required to file FDA 3514?
How to fill out FDA 3514?
What is the purpose of FDA 3514?
What information must be reported on FDA 3514?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

























