Get the free Immunoassay SOP - dctd cancer
Show details
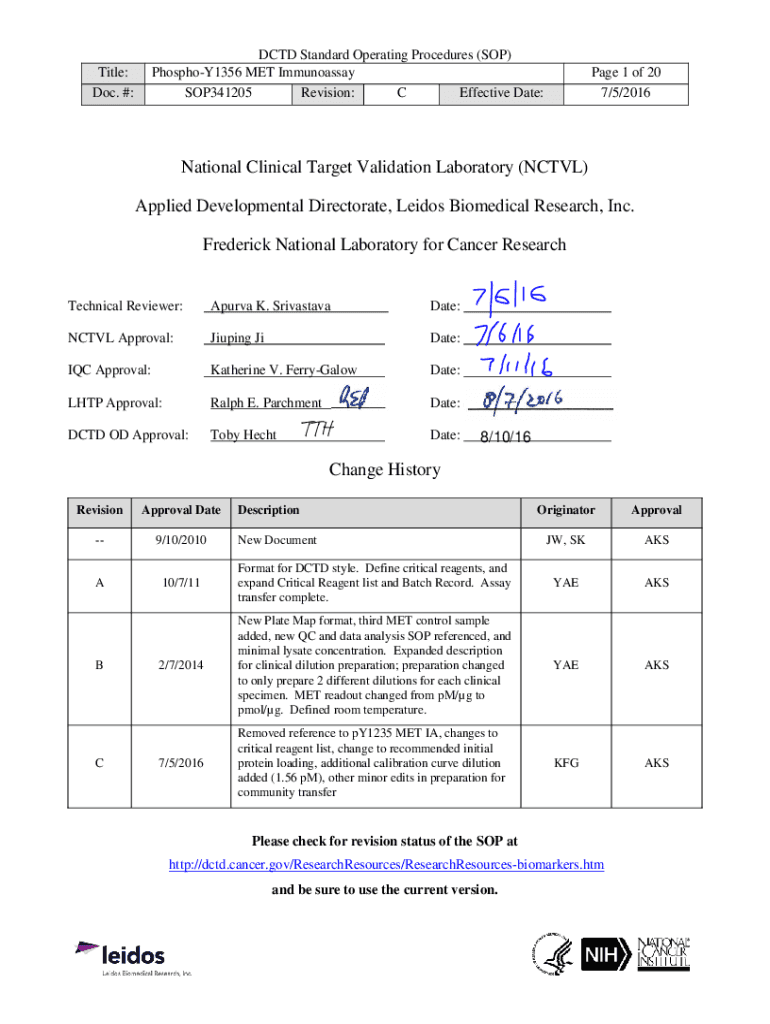
DCD Standard Operating Procedures (SOP) PhosphoY1356 MET Immunoassay SOP341205 Revision: C Effective Date:Title: Doc. #:Page 1 of 20 7/5/2016National Clinical Target Validation Laboratory (CTV) Applied
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign immunoassay sop - dctd

Edit your immunoassay sop - dctd form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your immunoassay sop - dctd form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing immunoassay sop - dctd online
Follow the guidelines below to take advantage of the professional PDF editor:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit immunoassay sop - dctd. Add and replace text, insert new objects, rearrange pages, add watermarks and page numbers, and more. Click Done when you are finished editing and go to the Documents tab to merge, split, lock or unlock the file.
4
Save your file. Choose it from the list of records. Then, shift the pointer to the right toolbar and select one of the several exporting methods: save it in multiple formats, download it as a PDF, email it, or save it to the cloud.
It's easier to work with documents with pdfFiller than you could have believed. You may try it out for yourself by signing up for an account.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out immunoassay sop - dctd

How to fill out immunoassay sop
01
Step 1: Start by gathering all the necessary materials such as the immunoassay sop template, a computer or paper for writing, and any relevant documents or information.
02
Step 2: Familiarize yourself with the immunoassay sop template by reading through it carefully, understanding the purpose and the required sections.
03
Step 3: Use a clear and concise language to describe the specific steps involved in performing the immunoassay procedure. Break down the process into smaller points for better readability and understanding.
04
Step 4: Include all necessary information such as equipment, reagents, and controls required for the immunoassay. Specify the quantities and concentrations if applicable.
05
Step 5: Provide instructions for any sample preparations or dilutions as well as the appropriate storage conditions.
06
Step 6: Consider including diagrams, images, or flowcharts to enhance the clarity of the instructions and make them visually appealing.
07
Step 7: Review and edit your immunoassay sop to ensure accuracy, completeness, and clarity. Make sure all steps are in the correct order and that there are no ambiguities or contradictions.
08
Step 8: Seek input or feedback from colleagues or experts in the field to ensure that the immunoassay sop is accurate, well-written, and easy to follow.
09
Step 9: Finalize the immunoassay sop by formatting it according to your organization's guidelines, adding a title, date, and any necessary signatures.
10
Step 10: Distribute the immunoassay sop to the relevant personnel or departments and ensure that it is accessible to everyone who needs it. Consider using digital platforms or a central repository for easy access and version control.
Who needs immunoassay sop?
01
The immunoassay sop is needed by laboratory technicians, scientists, or any personnel involved in the performance of immunoassay procedures.
02
Quality control managers or professionals responsible for ensuring the accuracy and consistency of immunoassay results may also require the immunoassay sop.
03
Regulatory bodies or agencies that oversee the practice of immunoassays may request the immunoassay sop for auditing, verification, or compliance purposes.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
Where do I find immunoassay sop - dctd?
It's simple using pdfFiller, an online document management tool. Use our huge online form collection (over 25M fillable forms) to quickly discover the immunoassay sop - dctd. Open it immediately and start altering it with sophisticated capabilities.
How do I complete immunoassay sop - dctd online?
Completing and signing immunoassay sop - dctd online is easy with pdfFiller. It enables you to edit original PDF content, highlight, blackout, erase and type text anywhere on a page, legally eSign your form, and much more. Create your free account and manage professional documents on the web.
Can I create an electronic signature for signing my immunoassay sop - dctd in Gmail?
Create your eSignature using pdfFiller and then eSign your immunoassay sop - dctd immediately from your email with pdfFiller's Gmail add-on. To keep your signatures and signed papers, you must create an account.
What is immunoassay sop?
Immunoassay sop is a standard operating procedure for conducting immunoassay tests to analyze levels of specific substances in biological samples.
Who is required to file immunoassay sop?
Laboratories and research facilities that conduct immunoassay tests are required to file immunoassay sop.
How to fill out immunoassay sop?
Immunoassay sop can be filled out by following the guidelines provided by regulatory agencies and ensuring all necessary information is included.
What is the purpose of immunoassay sop?
The purpose of immunoassay sop is to ensure standardized procedures are followed when conducting immunoassay tests, to maintain accuracy and reliability of results.
What information must be reported on immunoassay sop?
Immunoassay sop should include details about the specific immunoassay test being conducted, the equipment and reagents used, the procedures followed, and the personnel involved.
Fill out your immunoassay sop - dctd online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Immunoassay Sop - Dctd is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.



















