Get the free vaccine clinical research: Topics by Science.gov
Show details
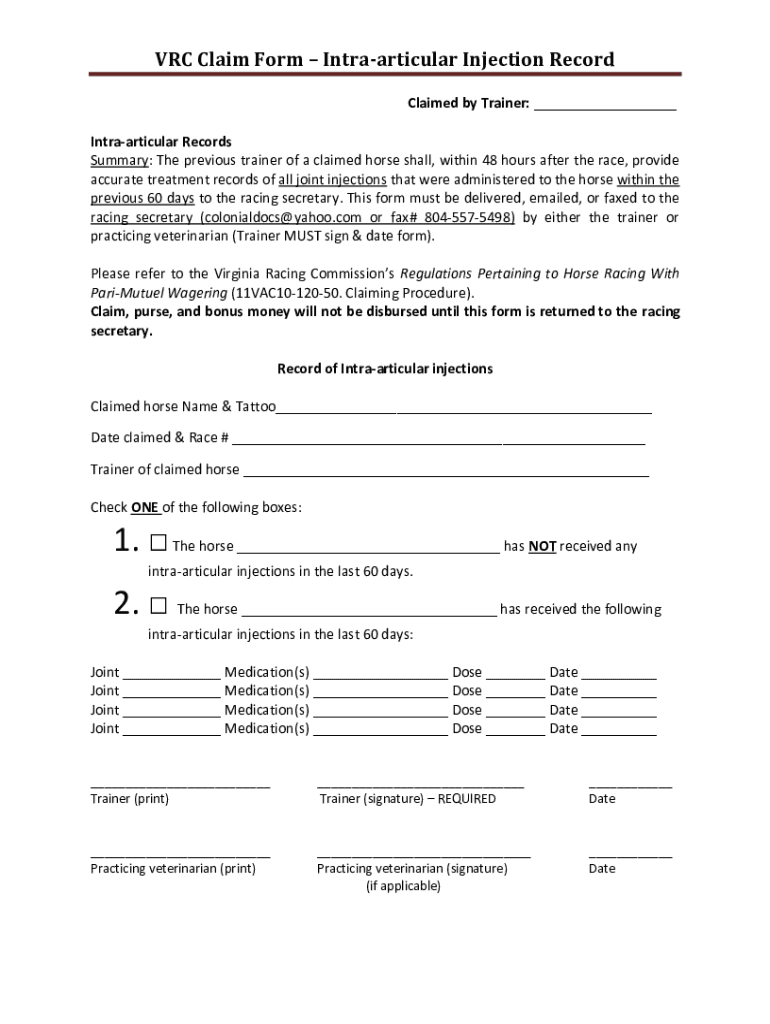
ARC Claim Form Intra-articular Injection Record Claimed by Trainer: ___ Intra-articular Records Summary: The previous trainer of a claimed horse shall, within 48 hours after the race, provide accurate
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign vaccine clinical research topics

Edit your vaccine clinical research topics form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your vaccine clinical research topics form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit vaccine clinical research topics online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Check your account. It's time to start your free trial.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit vaccine clinical research topics. Rearrange and rotate pages, add new and changed texts, add new objects, and use other useful tools. When you're done, click Done. You can use the Documents tab to merge, split, lock, or unlock your files.
4
Save your file. Choose it from the list of records. Then, shift the pointer to the right toolbar and select one of the several exporting methods: save it in multiple formats, download it as a PDF, email it, or save it to the cloud.
With pdfFiller, it's always easy to work with documents.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out vaccine clinical research topics

How to fill out vaccine clinical research topics
01
Start by understanding the purpose of the vaccine clinical research topics. Determine the specific objectives or outcomes you want to achieve with the research.
02
Conduct a thorough review of existing literature and research studies related to vaccine clinical research topics. This will help you identify the current gaps or areas that need further investigation.
03
Develop a research plan outlining the research design, methodology, and data collection methods. This plan should be aligned with ethical guidelines and regulations governing vaccine clinical research.
04
Identify the target population for your research. Determine the criteria for selecting participants who will be involved in the study.
05
Obtain the necessary approvals and permissions from relevant ethical review boards or regulatory bodies before proceeding with the research.
06
Collect and analyze data following the proposed research plan. Ensure data integrity and accuracy by implementing proper data management strategies.
07
Interpret the findings based on the collected data and draw appropriate conclusions. Analyze the implications and significance of the findings for vaccine development and public health.
08
Prepare a comprehensive report or manuscript summarizing the research process, results, and recommendations. Follow the guidelines of the target journal or publication for formatting and submission.
09
Disseminate and share the research findings through conferences, scientific meetings, or peer-reviewed publications. Collaborate with fellow researchers and experts in the field to promote knowledge exchange.
10
Periodically review and update the research topics based on emerging trends, technological advancements, or new findings in the field of vaccine clinical research.
Who needs vaccine clinical research topics?
01
Researchers and scientists working in the field of vaccine development and public health.
02
Pharmaceutical companies conducting clinical trials for vaccine candidates.
03
Health organizations and regulatory bodies responsible for monitoring and approving vaccines.
04
Medical professionals involved in vaccine administration and patient care.
05
Academic institutions and universities offering research programs in vaccine sciences.
06
Policy-makers and government agencies responsible for formulating public health strategies and vaccination campaigns.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I get vaccine clinical research topics?
It’s easy with pdfFiller, a comprehensive online solution for professional document management. Access our extensive library of online forms (over 25M fillable forms are available) and locate the vaccine clinical research topics in a matter of seconds. Open it right away and start customizing it using advanced editing features.
How do I fill out the vaccine clinical research topics form on my smartphone?
You can easily create and fill out legal forms with the help of the pdfFiller mobile app. Complete and sign vaccine clinical research topics and other documents on your mobile device using the application. Visit pdfFiller’s webpage to learn more about the functionalities of the PDF editor.
How do I complete vaccine clinical research topics on an iOS device?
Download and install the pdfFiller iOS app. Then, launch the app and log in or create an account to have access to all of the editing tools of the solution. Upload your vaccine clinical research topics from your device or cloud storage to open it, or input the document URL. After filling out all of the essential areas in the document and eSigning it (if necessary), you may save it or share it with others.
What is vaccine clinical research topics?
Vaccine clinical research topics include safety and efficacy studies, immunogenicity assessments, and post-licensure surveillance.
Who is required to file vaccine clinical research topics?
Pharmaceutical companies developing vaccines are required to file vaccine clinical research topics with regulatory authorities.
How to fill out vaccine clinical research topics?
Vaccine clinical research topics should be filled out by providing detailed information on the vaccine candidate, study design, endpoints, patient population, and safety monitoring.
What is the purpose of vaccine clinical research topics?
The purpose of vaccine clinical research topics is to ensure the safety and efficacy of vaccines before they are approved for use in the general population.
What information must be reported on vaccine clinical research topics?
Vaccine clinical research topics must include data on preclinical studies, clinical trial results, adverse events, and ongoing surveillance.
Fill out your vaccine clinical research topics online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Vaccine Clinical Research Topics is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.



















