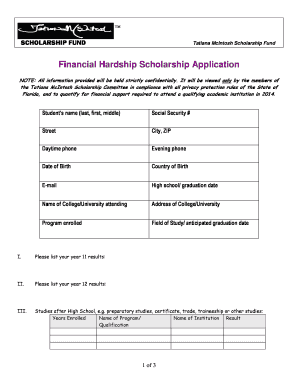
Get the free First-in-man treatment of severe blood pressure variability with ... - dnr wi
Show details
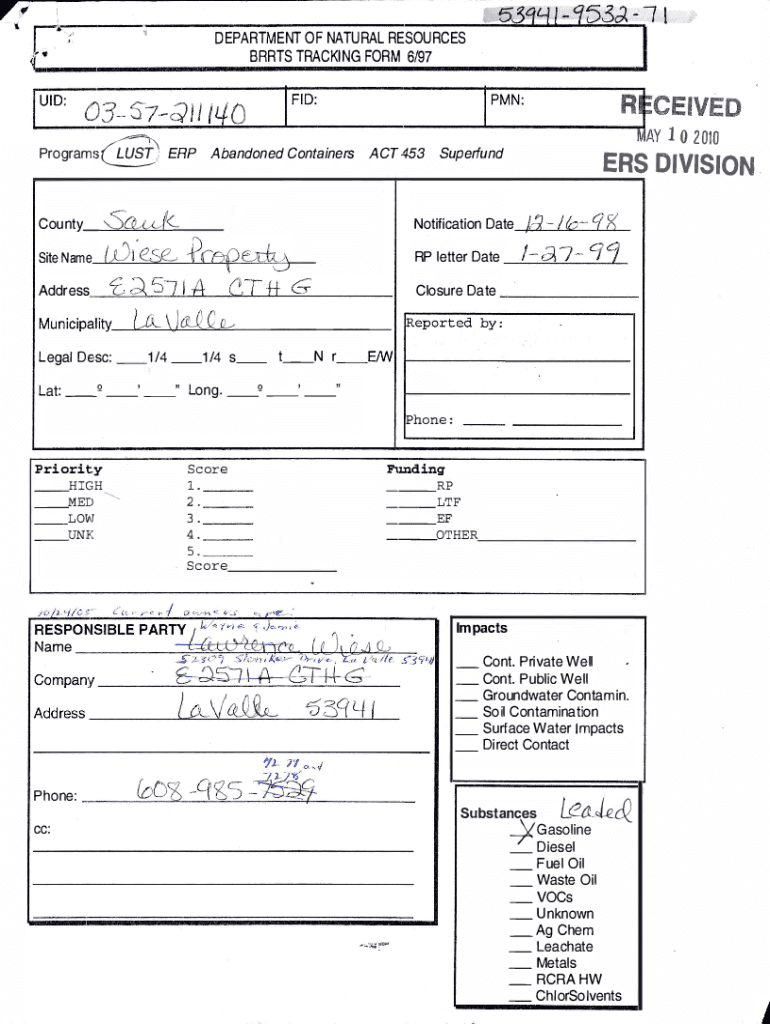
DEPARTMENT OF NATURAL RESOURCES PARTS TRACKING FORM 6/97I03 57QIIIL/0U IDF ID:53IR E C FIVE D nay 1 0 2010PMN:L. ;;;; ;, .:....: :::;,.z....:. . . ProgramsERPAbandoned ContainersSa&J(Site Name f.
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign first-in-man treatment of severe

Edit your first-in-man treatment of severe form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your first-in-man treatment of severe form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit first-in-man treatment of severe online
Use the instructions below to start using our professional PDF editor:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit first-in-man treatment of severe. Rearrange and rotate pages, add new and changed texts, add new objects, and use other useful tools. When you're done, click Done. You can use the Documents tab to merge, split, lock, or unlock your files.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
With pdfFiller, dealing with documents is always straightforward. Try it right now!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out first-in-man treatment of severe

How to fill out first-in-man treatment of severe
01
Gather all necessary medical records and documents related to the patient's condition.
02
Consult with the patient's multidisciplinary medical team, including specialists and experts in the field.
03
Conduct a comprehensive assessment and evaluation of the patient's medical history, current condition, and potential risks.
04
Develop a detailed treatment plan that outlines the goals, procedures, and expected outcomes of the first-in-man treatment.
05
Obtain informed consent from the patient or their legal representative, emphasizing the experimental nature and potential risks of the treatment.
06
Ensure that the medical facility has the necessary resources, infrastructure, and expertise to provide appropriate care during the treatment process.
07
Follow all ethical guidelines, regulatory requirements, and institutional protocols governing first-in-man treatment.
08
Monitor the patient closely during and after the treatment, paying attention to any adverse reactions or complications.
09
Continuously evaluate and document the patient's progress, making adjustments to the treatment plan as necessary.
10
Communicate and collaborate with the patient, their family, and the medical team to ensure clear and effective communication throughout the treatment process.
Who needs first-in-man treatment of severe?
01
First-in-man treatment of severe is typically recommended for patients who have exhausted all standard treatment options without success.
02
These patients may have severe, life-threatening conditions or diseases for which there are no approved or effective treatments available.
03
First-in-man treatment can be considered for patients who are willing to participate in experimental treatments and understand the potential risks involved.
04
It is crucial to assess each patient's individual case, weighing the potential benefits against the risks and ensuring that the patient's consent is obtained.
05
Ultimately, the decision to pursue first-in-man treatment should be made in consultation with the patient's medical team, who can provide expert guidance based on the patient's specific condition and circumstances.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I get first-in-man treatment of severe?
The premium version of pdfFiller gives you access to a huge library of fillable forms (more than 25 million fillable templates). You can download, fill out, print, and sign them all. State-specific first-in-man treatment of severe and other forms will be easy to find in the library. Find the template you need and use advanced editing tools to make it your own.
How do I make edits in first-in-man treatment of severe without leaving Chrome?
Install the pdfFiller Google Chrome Extension to edit first-in-man treatment of severe and other documents straight from Google search results. When reading documents in Chrome, you may edit them. Create fillable PDFs and update existing PDFs using pdfFiller.
Can I edit first-in-man treatment of severe on an Android device?
You can edit, sign, and distribute first-in-man treatment of severe on your mobile device from anywhere using the pdfFiller mobile app for Android; all you need is an internet connection. Download the app and begin streamlining your document workflow from anywhere.
What is first-in-man treatment of severe?
First-in-man treatment of severe refers to the first time a new drug or medical device is tested on humans for the treatment of a severe condition.
Who is required to file first-in-man treatment of severe?
The researchers or pharmaceutical companies conducting the clinical trial are required to file the first-in-man treatment of severe.
How to fill out first-in-man treatment of severe?
To fill out the first-in-man treatment of severe, researchers must provide detailed information about the study design, potential risks and benefits, and informed consent procedures.
What is the purpose of first-in-man treatment of severe?
The purpose of first-in-man treatment of severe is to assess the safety and efficacy of a new drug or medical device in humans for the treatment of a severe condition.
What information must be reported on first-in-man treatment of severe?
Information such as study protocol, potential risks and benefits, informed consent process, and data collection procedures must be reported on first-in-man treatment of severe.
Fill out your first-in-man treatment of severe online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

First-In-Man Treatment Of Severe is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.