Get the free Registration & Randomisation forms.doc
Show details
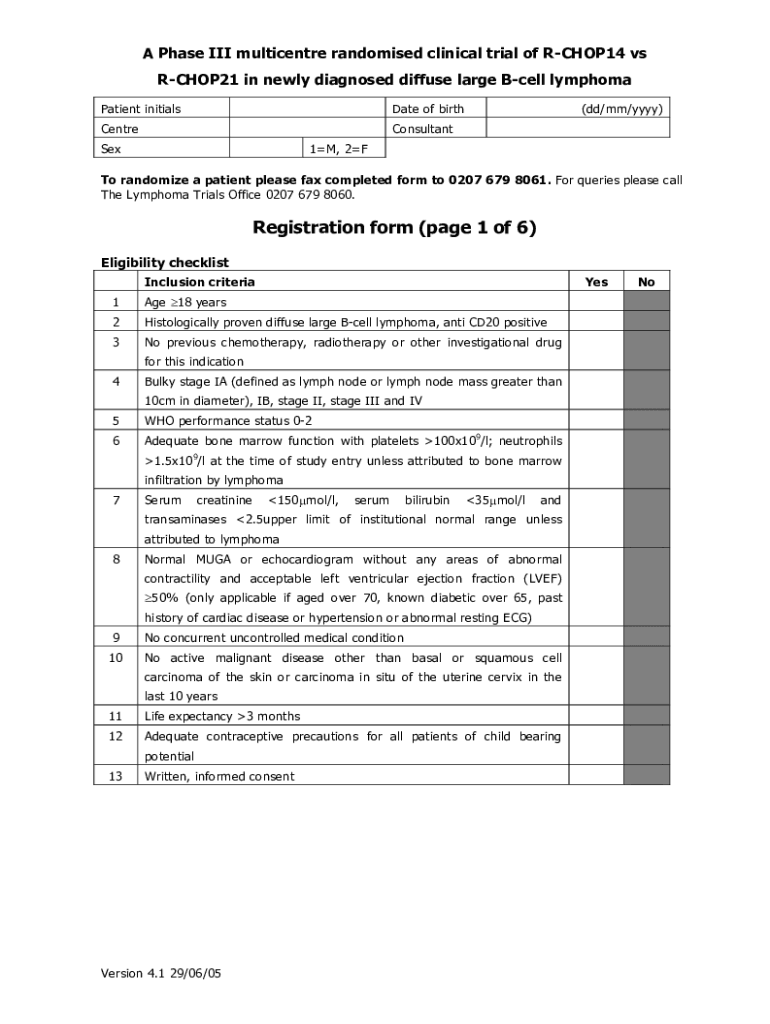
A Phase III multigenre randomized clinical trial of RCHOP14 vs RCHOP21 in newly diagnosed diffuse large Cell lymphoma Patient initialsDate of birthCentreConsultantSex(dd/mm/YYY)1M, 2FTo randomize
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign registration ampamp randomisation formsdoc

Edit your registration ampamp randomisation formsdoc form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your registration ampamp randomisation formsdoc form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit registration ampamp randomisation formsdoc online
Follow the steps below to use a professional PDF editor:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit registration ampamp randomisation formsdoc. Rearrange and rotate pages, insert new and alter existing texts, add new objects, and take advantage of other helpful tools. Click Done to apply changes and return to your Dashboard. Go to the Documents tab to access merging, splitting, locking, or unlocking functions.
4
Save your file. Choose it from the list of records. Then, shift the pointer to the right toolbar and select one of the several exporting methods: save it in multiple formats, download it as a PDF, email it, or save it to the cloud.
With pdfFiller, dealing with documents is always straightforward.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out registration ampamp randomisation formsdoc

How to fill out registration ampamp randomisation formsdoc
01
To fill out the registration & randomisation form.doc, follow these steps:
1. Open the form.doc file using a word processing software like Microsoft Word.
02
Review the instructions provided at the beginning of the form to understand the purpose and requirements for filling it out.
03
Begin filling out the form by entering the required information in the appropriate fields. Make sure to provide accurate and complete details.
04
Pay attention to any specific formatting or data entry guidelines stated in the instructions. Follow them accordingly.
05
Double-check all the entered information for any errors or omissions. It's important to ensure the form is correctly and completely filled out.
06
Save the filled-out form on your computer or device. Consider using a clear and identifiable name for the saved file.
07
If required, print a hard copy of the form for record-keeping or submission.
08
Submit the completed form according to the specified instructions or deliver it to the designated recipient.
09
Keep a copy of the submitted form for your records, if needed, and ensure data privacy.
10
It is recommended to consult any additional guidelines or resources provided alongside the form for further assistance.
Who needs registration ampamp randomisation formsdoc?
01
Registration & randomisation forms.doc may be required by researchers, study coordinators, or project teams involved in conducting clinical trials or scientific research.
02
These forms are used to register and randomly assign participants to different groups or treatments within a study or experiment.
03
They help ensure proper documentation, organization, and control in the experimental process. The need for such forms depends on the specific research protocol and study design being implemented.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I send registration ampamp randomisation formsdoc for eSignature?
When you're ready to share your registration ampamp randomisation formsdoc, you can send it to other people and get the eSigned document back just as quickly. Share your PDF by email, fax, text message, or USPS mail. You can also notarize your PDF on the web. You don't have to leave your account to do this.
How can I edit registration ampamp randomisation formsdoc on a smartphone?
You can do so easily with pdfFiller’s applications for iOS and Android devices, which can be found at the Apple Store and Google Play Store, respectively. Alternatively, you can get the app on our web page: https://edit-pdf-ios-android.pdffiller.com/. Install the application, log in, and start editing registration ampamp randomisation formsdoc right away.
How can I fill out registration ampamp randomisation formsdoc on an iOS device?
pdfFiller has an iOS app that lets you fill out documents on your phone. A subscription to the service means you can make an account or log in to one you already have. As soon as the registration process is done, upload your registration ampamp randomisation formsdoc. You can now use pdfFiller's more advanced features, like adding fillable fields and eSigning documents, as well as accessing them from any device, no matter where you are in the world.
What is registration ampamp randomisation formsdoc?
Registration and randomisation forms document is a formal record used in research studies to track participant registration and allocation to treatment groups.
Who is required to file registration ampamp randomisation formsdoc?
Researchers and institutions conducting clinical trials or studies involving human participants are required to file this documentation.
How to fill out registration ampamp randomisation formsdoc?
To fill out the form, researchers must provide detailed information about the study, including participant criteria, randomisation methods, and consent processes.
What is the purpose of registration ampamp randomisation formsdoc?
The purpose is to ensure transparency, accountability, and proper tracking of participants in clinical trials for reliability and validity of results.
What information must be reported on registration ampamp randomisation formsdoc?
Information required includes study title, objectives, methods of randomisation, inclusion/exclusion criteria, and participant demographics.
Fill out your registration ampamp randomisation formsdoc online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Registration Ampamp Randomisation Formsdoc is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.


















