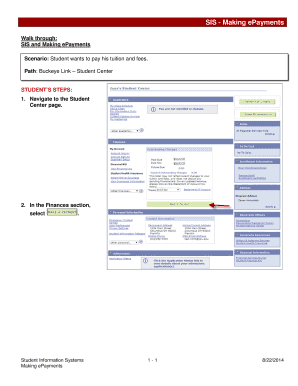
Scientific Update Preclinical Safety Assessment and Mitigation Strategies in Drug Discovery 2019-2025 free printable template
Show details
PRECLINICAL SAFETY
ASSESSMENT AND
MITIGATION STRATEGIES
IN DRUG DISCOVERY
1920 MARCH 2019
Boston, MA USA
Boston Metro
Meeting Centered
COURSE! An 11/2-day course given by
Dr Bryan H. Nonprofessional
pdfFiller is not affiliated with any government organization
Get, Create, Make and Sign Scientific Update Preclinical Safety Assessment and

Edit your Scientific Update Preclinical Safety Assessment and form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your Scientific Update Preclinical Safety Assessment and form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit Scientific Update Preclinical Safety Assessment and online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Upload a document. Select Add New on your Dashboard and transfer a file into the system in one of the following ways: by uploading it from your device or importing from the cloud, web, or internal mail. Then, click Start editing.
3
Edit Scientific Update Preclinical Safety Assessment and. Replace text, adding objects, rearranging pages, and more. Then select the Documents tab to combine, divide, lock or unlock the file.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
Dealing with documents is simple using pdfFiller. Try it right now!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
Scientific Update Preclinical Safety Assessment and Mitigation Strategies in Drug Discovery Form Versions
Version
Form Popularity
Fillable & printabley
4.8 Satisfied (92 Votes)
4.2 Satisfied (62 Votes)
How to fill out Scientific Update Preclinical Safety Assessment and

How to fill out Scientific Update Preclinical Safety Assessment and Mitigation
01
Gather necessary documentation related to the preclinical studies.
02
Identify potential safety concerns based on previous data and literature.
03
Complete sections detailing the study design, including animal models and methodologies used.
04
Provide information on dosing, duration of exposure, and endpoints assessed.
05
Analyze and summarize adverse findings from the studies.
06
Assess risk factors and propose mitigation strategies for identified risks.
07
Compile all data in a clear and organized manner following the specified format.
Who needs Scientific Update Preclinical Safety Assessment and Mitigation?
01
Researchers conducting preclinical studies in drug development.
02
Regulatory agencies assessing safety for new therapeutic agents.
03
Pharmaceutical companies preparing for IND (Investigational New Drug) applications.
04
Contract research organizations (CROs) involved in safety assessments.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I edit Scientific Update Preclinical Safety Assessment and online?
With pdfFiller, the editing process is straightforward. Open your Scientific Update Preclinical Safety Assessment and in the editor, which is highly intuitive and easy to use. There, you’ll be able to blackout, redact, type, and erase text, add images, draw arrows and lines, place sticky notes and text boxes, and much more.
Can I edit Scientific Update Preclinical Safety Assessment and on an iOS device?
You certainly can. You can quickly edit, distribute, and sign Scientific Update Preclinical Safety Assessment and on your iOS device with the pdfFiller mobile app. Purchase it from the Apple Store and install it in seconds. The program is free, but in order to purchase a subscription or activate a free trial, you must first establish an account.
How can I fill out Scientific Update Preclinical Safety Assessment and on an iOS device?
In order to fill out documents on your iOS device, install the pdfFiller app. Create an account or log in to an existing one if you have a subscription to the service. Once the registration process is complete, upload your Scientific Update Preclinical Safety Assessment and. You now can take advantage of pdfFiller's advanced functionalities: adding fillable fields and eSigning documents, and accessing them from any device, wherever you are.
What is Scientific Update Preclinical Safety Assessment and Mitigation?
Scientific Update Preclinical Safety Assessment and Mitigation refers to a structured process to evaluate the safety of compounds before clinical trials. It aims to identify potential risks through preclinical studies and to develop strategies to mitigate those risks.
Who is required to file Scientific Update Preclinical Safety Assessment and Mitigation?
Typically, organizations and sponsors planning to conduct clinical trials are required to file Scientific Update Preclinical Safety Assessment and Mitigation. This includes pharmaceutical companies, biotech firms, and research institutions.
How to fill out Scientific Update Preclinical Safety Assessment and Mitigation?
To fill out the Scientific Update Preclinical Safety Assessment and Mitigation, one must provide detailed information on the preclinical studies conducted, data on the safety assessments, risk factors identified, and proposed mitigation strategies. Guidelines from regulatory bodies should be followed to ensure compliance.
What is the purpose of Scientific Update Preclinical Safety Assessment and Mitigation?
The purpose of Scientific Update Preclinical Safety Assessment and Mitigation is to ensure that the safety of investigational drugs is thoroughly evaluated prior to human trials, thereby protecting participants and ensuring regulatory compliance.
What information must be reported on Scientific Update Preclinical Safety Assessment and Mitigation?
Information that must be reported includes study protocols, results of toxicity studies, pharmacokinetic and pharmacodynamic data, risk assessments, and any proposed strategies for risk mitigation.
Fill out your Scientific Update Preclinical Safety Assessment and online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Scientific Update Preclinical Safety Assessment And is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.