Get the free 222 Laboratory Improement Programs Order Form
Show details
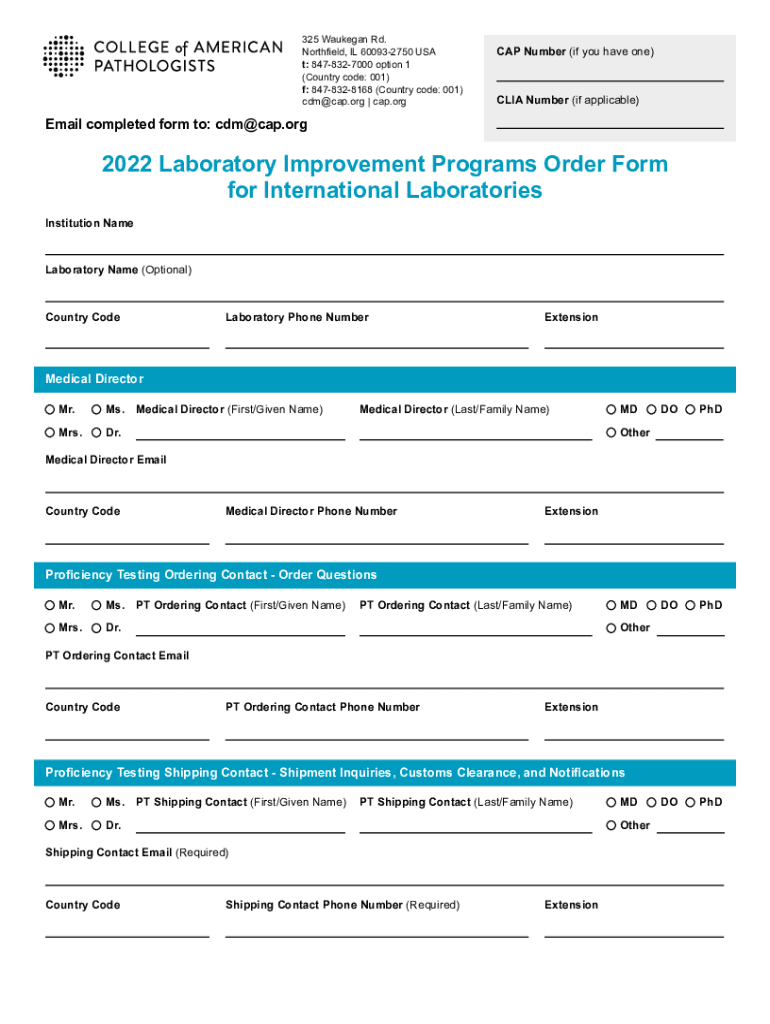
325 Waukegan Rd. Northfield, IL 600932750 USA t: 8478327000 option 1 (Country code: 001) f: 8478328168 (Country code: 001) CDM cap.org cap.org2022 Laboratory Improvement Programs Order Form for International
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign 222 laboratory improement programs

Edit your 222 laboratory improement programs form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your 222 laboratory improement programs form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit 222 laboratory improement programs online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit 222 laboratory improement programs. Rearrange and rotate pages, add new and changed texts, add new objects, and use other useful tools. When you're done, click Done. You can use the Documents tab to merge, split, lock, or unlock your files.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
pdfFiller makes dealing with documents a breeze. Create an account to find out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out 222 laboratory improement programs

How to fill out 222 laboratory improement programs
01
Start by gathering all the necessary information and documents related to the laboratory improvement programs.
02
Review the requirements and guidelines provided for filling out the form 222.
03
Fill out the form in a point-by-point manner, ensuring accuracy and completeness.
04
Provide detailed explanations and justifications for each improvement program idea.
05
Include any supporting documentation or evidence to strengthen your proposals.
06
Double-check the filled-out form for any errors or missing information.
07
Make sure to submit the completed form to the appropriate authority or department.
Who needs 222 laboratory improement programs?
01
Laboratories and organizations looking to enhance their operations and quality standards can benefit from 222 laboratory improvement programs.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I edit 222 laboratory improement programs from Google Drive?
You can quickly improve your document management and form preparation by integrating pdfFiller with Google Docs so that you can create, edit and sign documents directly from your Google Drive. The add-on enables you to transform your 222 laboratory improement programs into a dynamic fillable form that you can manage and eSign from any internet-connected device.
How do I edit 222 laboratory improement programs online?
pdfFiller not only allows you to edit the content of your files but fully rearrange them by changing the number and sequence of pages. Upload your 222 laboratory improement programs to the editor and make any required adjustments in a couple of clicks. The editor enables you to blackout, type, and erase text in PDFs, add images, sticky notes and text boxes, and much more.
How do I edit 222 laboratory improement programs on an Android device?
You can make any changes to PDF files, such as 222 laboratory improement programs, with the help of the pdfFiller mobile app for Android. Edit, sign, and send documents right from your mobile device. Install the app and streamline your document management wherever you are.
What is 222 laboratory improvement programs?
The 222 laboratory improvement programs refer to regulated programs that provide guidelines for maintaining and improving laboratory quality and compliance with standards.
Who is required to file 222 laboratory improvement programs?
Laboratories that handle certain regulated substances or that require certification for quality assurance purposes are required to file the 222 laboratory improvement programs.
How to fill out 222 laboratory improvement programs?
To fill out the 222 laboratory improvement programs, laboratories must complete the specific forms provided by the regulatory authority, including details about laboratory practices, compliance measures, and quality control procedures.
What is the purpose of 222 laboratory improvement programs?
The purpose of the 222 laboratory improvement programs is to ensure that laboratories adhere to high standards of quality, safety, and compliance, thus safeguarding public health.
What information must be reported on 222 laboratory improvement programs?
The information that must be reported includes laboratory policies, procedures, quality control measures, staff qualifications, and any incidents or compliance issues.
Fill out your 222 laboratory improement programs online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

222 Laboratory Improement Programs is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.


















