Get the free Precertification-form-drugs-biologics-part-b ...
Show details
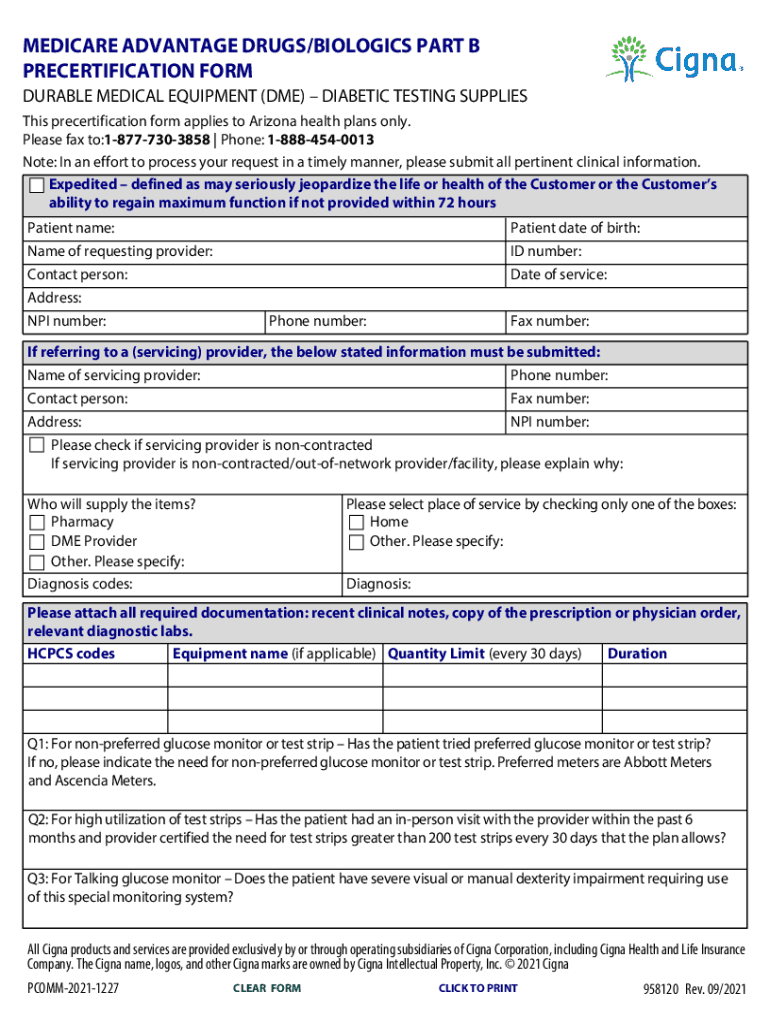
MEDICARE ADVANTAGE DRUGS/BIOLOGICS PART B RECERTIFICATION FORM DURABLE MEDICAL EQUIPMENT (DME) DIABETIC TESTING SUPPLIES This recertification form applies to Arizona health plans only. Please fax
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign precertification-form-drugs-biologics-part-b

Edit your precertification-form-drugs-biologics-part-b form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your precertification-form-drugs-biologics-part-b form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing precertification-form-drugs-biologics-part-b online
To use the professional PDF editor, follow these steps:
1
Create an account. Begin by choosing Start Free Trial and, if you are a new user, establish a profile.
2
Upload a file. Select Add New on your Dashboard and upload a file from your device or import it from the cloud, online, or internal mail. Then click Edit.
3
Edit precertification-form-drugs-biologics-part-b. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Save your file. Select it from your records list. Then, click the right toolbar and select one of the various exporting options: save in numerous formats, download as PDF, email, or cloud.
With pdfFiller, it's always easy to work with documents.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out precertification-form-drugs-biologics-part-b

How to fill out precertification-form-drugs-biologics-part-b
01
To fill out the precertification-form-drugs-biologics-part-b, follow these steps:
02
Start by filling out the patient's personal information, including their name, date of birth, and address.
03
Provide the patient's insurance information, including the insurance company's name, policy number, and group number.
04
Indicate the type of drug or biologic that requires precertification.
05
Provide detailed information about the drug or biologic, including the name, dosage, frequency of administration, and reason for use.
06
Document any prior treatment the patient has undergone for the same condition.
07
Include any relevant medical records or supporting documentation to justify the need for precertification.
08
Sign and date the form before submitting it to the appropriate department or insurance provider.
09
Keep a copy of the completed form for your records.
Who needs precertification-form-drugs-biologics-part-b?
01
Precertification-form-drugs-biologics-part-b is needed for individuals who require authorization from their insurance provider before receiving certain types of drugs or biologics.
02
Patients with specific health conditions or those who require expensive medications may need to fill out this form to ensure coverage and reimbursement for their prescribed treatments.
03
Insurance providers often require precertification for drugs or biologics that may be subject to certain utilization management or cost-control measures.
04
It is recommended to consult with your healthcare provider or insurance company to determine if you need to fill out the precertification-form-drugs-biologics-part-b for your specific medication or treatment.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I get precertification-form-drugs-biologics-part-b?
The pdfFiller premium subscription gives you access to a large library of fillable forms (over 25 million fillable templates) that you can download, fill out, print, and sign. In the library, you'll have no problem discovering state-specific precertification-form-drugs-biologics-part-b and other forms. Find the template you want and tweak it with powerful editing tools.
Can I create an eSignature for the precertification-form-drugs-biologics-part-b in Gmail?
With pdfFiller's add-on, you may upload, type, or draw a signature in Gmail. You can eSign your precertification-form-drugs-biologics-part-b and other papers directly in your mailbox with pdfFiller. To preserve signed papers and your personal signatures, create an account.
How do I complete precertification-form-drugs-biologics-part-b on an iOS device?
Get and install the pdfFiller application for iOS. Next, open the app and log in or create an account to get access to all of the solution’s editing features. To open your precertification-form-drugs-biologics-part-b, upload it from your device or cloud storage, or enter the document URL. After you complete all of the required fields within the document and eSign it (if that is needed), you can save it or share it with others.
What is precertification-form-drugs-biologics-part-b?
Precertification-form-drugs-biologics-part-b is a form that needs to be filed for pre-approval of certain drugs and biologics under Part B of the Medicare program.
Who is required to file precertification-form-drugs-biologics-part-b?
Healthcare providers and facilities that wish to administer certain drugs and biologics under Part B of the Medicare program are required to file precertification-form-drugs-biologics-part-b.
How to fill out precertification-form-drugs-biologics-part-b?
Precertification-form-drugs-biologics-part-b can be filled out electronically through the Medicare Administrative Contractor (MAC) portal or by submitting a paper form with all required information.
What is the purpose of precertification-form-drugs-biologics-part-b?
The purpose of precertification-form-drugs-biologics-part-b is to ensure that the drugs and biologics to be administered under Part B of the Medicare program meet the necessary criteria for coverage and reimbursement.
What information must be reported on precertification-form-drugs-biologics-part-b?
The precertification-form-drugs-biologics-part-b requires information such as the drug or biologic name, dosage, administration route, diagnosis codes, provider information, and supporting documentation.
Fill out your precertification-form-drugs-biologics-part-b online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Precertification-Form-Drugs-Biologics-Part-B is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.