Get the free Pediatric Heart Transplant Study ID# P FORM 1205 Pre ...
Show details
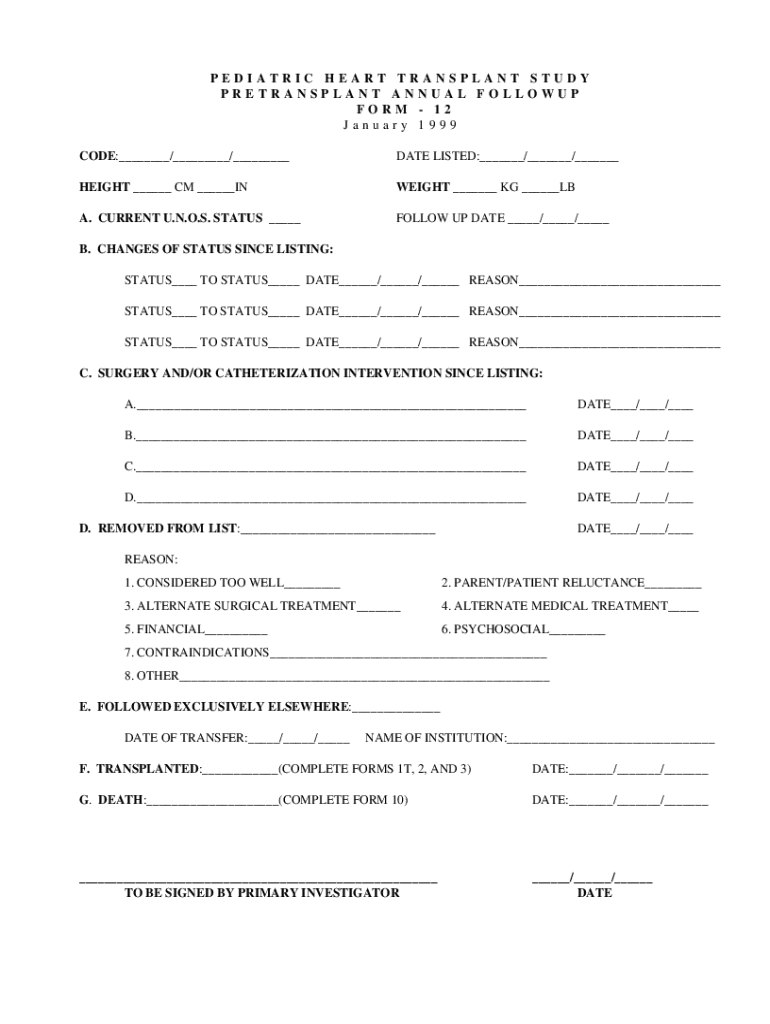
PEDIATRIC HEART TRANSPLANT STUDY TRANSPLANT ANNUAL FOLLOWUP FORM 12 January 1999 CODE: / / DATE LISTED: / / HEIGHT CM WEIGHT KG LBA. CURRENT U.N.O.S. STATUS FOLLOW-UP DATE / / B. CHANGES OF STATUS
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign pediatric heart transplant study

Edit your pediatric heart transplant study form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your pediatric heart transplant study form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing pediatric heart transplant study online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Upload a document. Select Add New on your Dashboard and transfer a file into the system in one of the following ways: by uploading it from your device or importing from the cloud, web, or internal mail. Then, click Start editing.
3
Edit pediatric heart transplant study. Add and change text, add new objects, move pages, add watermarks and page numbers, and more. Then click Done when you're done editing and go to the Documents tab to merge or split the file. If you want to lock or unlock the file, click the lock or unlock button.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
pdfFiller makes dealing with documents a breeze. Create an account to find out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out pediatric heart transplant study

How to fill out pediatric heart transplant study
01
Begin by gathering all the necessary medical records of the pediatric patient, including previous diagnostic tests, imaging results, and any relevant medical history.
02
Familiarize yourself with the pediatric heart transplant study protocol and the specific requirements and guidelines.
03
Ensure that you have obtained proper consent from the patient's legal guardian or parent to participate in the study.
04
Follow the study's instructions for filling out the required forms and questionnaires, providing accurate and detailed information about the patient's condition and medical history.
05
Include any relevant test results, lab reports, and imaging studies that are required as part of the study.
06
Pay close attention to any specific instructions regarding medications and dosages for the patient during the study period.
07
Keep thorough and organized documentation of the study process, including dates of participation, any adverse events or complications, and the patient's overall progress.
08
Submit the completed study forms, questionnaires, and required documents to the designated study coordinator or institution, following their specified submission guidelines.
Who needs pediatric heart transplant study?
01
Pediatric heart transplant studies are typically conducted for children who are in need of a heart transplant due to severe cardiac conditions or congenital heart defects.
02
This study is beneficial for patients who are potential candidates for heart transplantation, as it helps to gather important data and improve medical understanding of pediatric heart transplants.
03
Pediatric heart transplant studies may also benefit medical professionals and researchers who are involved in the field of pediatric cardiology and transplantation, as the findings can contribute to advancements in treatment and care for pediatric heart patients.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I complete pediatric heart transplant study online?
pdfFiller makes it easy to finish and sign pediatric heart transplant study online. It lets you make changes to original PDF content, highlight, black out, erase, and write text anywhere on a page, legally eSign your form, and more, all from one place. Create a free account and use the web to keep track of professional documents.
How do I fill out pediatric heart transplant study using my mobile device?
Use the pdfFiller mobile app to fill out and sign pediatric heart transplant study. Visit our website (https://edit-pdf-ios-android.pdffiller.com/) to learn more about our mobile applications, their features, and how to get started.
How do I complete pediatric heart transplant study on an Android device?
Use the pdfFiller Android app to finish your pediatric heart transplant study and other documents on your Android phone. The app has all the features you need to manage your documents, like editing content, eSigning, annotating, sharing files, and more. At any time, as long as there is an internet connection.
What is pediatric heart transplant study?
Pediatric heart transplant study is a research study focusing on heart transplants in children.
Who is required to file pediatric heart transplant study?
Medical professionals and researchers involved in pediatric heart transplants are required to file pediatric heart transplant study.
How to fill out pediatric heart transplant study?
Pediatric heart transplant study can be filled out by collecting data and information related to pediatric heart transplants and entering it into the designated form.
What is the purpose of pediatric heart transplant study?
The purpose of pediatric heart transplant study is to gather information and improve outcomes for children undergoing heart transplants.
What information must be reported on pediatric heart transplant study?
Information such as patient demographics, transplant procedure details, follow-up care, and outcomes must be reported on pediatric heart transplant study.
Fill out your pediatric heart transplant study online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Pediatric Heart Transplant Study is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















